Umeclidinium bromide versus placebo for people with chronic obstructive pulmonary disease (COPD)
- PMID: 28631387
- PMCID: PMC6481854
- DOI: 10.1002/14651858.CD011897.pub2
Umeclidinium bromide versus placebo for people with chronic obstructive pulmonary disease (COPD)
Abstract
Background: People with chronic obstructive pulmonary disease (COPD) have poor quality of life, reduced survival, and accelerated decline in lung function, especially associated with acute exacerbations, leading to high healthcare costs. Long-acting bronchodilators are the mainstay of treatment for symptomatic improvement, and umeclidinium is one of the new long-acting muscarinic antagonists approved for treatment of patients with stable COPD.
Objectives: To assess the efficacy and safety of umeclidinium bromide versus placebo for people with stable COPD.
Search methods: We searched the Cochrane Airways Group Specialised Register (CAGR), ClinicalTrials.gov, the World Health Organization (WHO) trials portal, and the GlaxoSmithKline (GSK) Clinical Study Register, using prespecified terms, as well as the reference lists of all identified studies. Searches are current to April 2017.
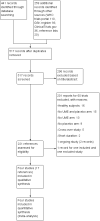
Selection criteria: We included randomised controlled trials (RCTs) of parallel design comparing umeclidinium bromide versus placebo in people with COPD, for at least 12 weeks.
Data collection and analysis: We used standard Cochrane methodological procedures. If we noted significant heterogeneity in the meta-analyses, we subgrouped studies by umeclidinium dose.
Main results: We included four studies of 12 to 52 weeks' duration, involving 3798 participants with COPD. Mean age of participants ranged from 60.1 to 64.6 years; most were males with baseline mean smoking pack-years of 39.2 to 52.3. They had moderate to severe COPD and baseline mean post-bronchodilator forced expiratory volume in one second (FEV1) ranging from 44.5% to 55.1% of predicted normal. As all studies were systematically conducted according to prespecified protocols, we assessed risk of selection, performance, detection, attrition, and reporting biases as low.Compared with those given placebo, participants in the umeclidinium group had a lesser likelihood of developing moderate exacerbations requiring a short course of steroids, antibiotics, or both (odds ratio (OR) 0.61, 95% confidence interval (CI) 0.46 to 0.80; four studies, N = 1922; GRADE: high), but not specifically requiring hospitalisations due to severe exacerbations (OR 0.86, 95% CI 0.25 to 2.92; four studies, N = 1922, GRADE: low). The number needed to treat for an additional beneficial outcome (NNTB) to prevent an acute exacerbation requiring steroids, antibiotics, or both was 18 (95% CI 13 to 37). Quality of life was better in the umeclidinium group (mean difference (MD) -4.79, 95% CI -8.84 to -0.75; three studies, N = 1119), and these participants had a significantly higher chance of achieving a minimal clinically important difference of at least four units in St George's Respiratory Questionnaire (SGRQ) total score compared with those in the placebo group (OR 1.45, 95% CI 1.16 to 1.82; three studies, N = 1397; GRADE: moderate). The NNTB to achieve one person with a clinically meaningful improvement was 11 (95% CI 7 to 29). The likelihood of all-cause mortality, non-fatal serious adverse events (OR 1.33; 95% CI 0.89 to 2.00; four studies, N = 1922, GRADE: moderate), and adverse events (OR 1.06, 95% CI 0.85 to 1.31; four studies, N = 1922; GRADE: moderate) did not differ between umeclidinium and placebo groups. The umeclidinium group demonstrated significantly greater improvement in change from baseline in trough FEV1 compared with the placebo group (MD 0.14, 95% CI 0.12 to 0.17; four studies, N = 1381; GRADE: high). Symptomatic improvement was more likely in the umeclidinium group than in the placebo group, as determined by Transitional Dyspnoea Index (TDI) focal score (MD 0.76, 95% CI 0.43 to 1.09; three studies, N = 1193), and the chance of achieving a minimal clinically important difference of at least one unit improvement was significantly higher with umeclidinium than with placebo (OR 1.71, 95% CI 1.37 to 2.15; three studies, N = 1141; GRADE: high). The NNTB to attain one person with clinically important symptomatic improvement was 8 (95% CI 5 to 14). The likelihood of rescue medication usage (change from baseline in the number of puffs per day) was significantly less for the umeclidinium group than for the placebo group (MD -0.45, 95% CI -0.76 to -0.14; four studies, N = 1531).
Authors' conclusions: Umeclidinium reduced acute exacerbations requiring steroids, antibiotics, or both, although no evidence suggests that it decreased the risk of hospital admission due to exacerbations. Moreover, umeclidinium demonstrated significant improvement in quality of life, lung function, and symptoms, along with lesser use of rescue medications. Studies reported no differences in adverse events, non-fatal serious adverse events, or mortality between umeclidinium and placebo groups; however, larger studies would yield a more precise estimate for these outcomes.
Conflict of interest statement
HN: none known.
AH: none known.
SM: none known.
We are conducting this systematic review for academic purposes.
Figures



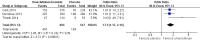
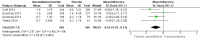
Update of
References
References to studies included in this review
Celli 2014 {published and unpublished data}
-
- Celli B, Crater G, Kilbride S, Mehta R, Tabberer M, Kalberg CJ, et al. Once‐daily umeclidinium/vilanterol 125/25 mug therapy in COPD. Chest 2014;145(5):981‐91. [CENTRAL: 988035; CRS: 4900126000012442; EMBASE: 2014313695] - PubMed
-
- Celli BR, Crater G, Kilbride S, Mehta R, Tabberer M, Kalberg CJ, et al. A 24‐week randomised, double‐blind, placebo‐controlled study of the efficacy and safety of once‐daily umeclidinium/vilanterol 125/25 mcg in COPD. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A2435. [CENTRAL: 870686; CRS: 4900100000087831]
-
- GSK573719+GW642444. A 24‐week, randomised, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of GSK573719/GW642444 inhalation powder and the individual components delivered once‐daily via a novel dry powder inhaler in subjects with chronic obstructive pulmonary disease. http://www.gsk‐clinicalstudyregister.com/files/113361/2404/gsk‐113361‐clinical‐study‐re... (first received 22 March 2011).
-
- Goyal N, Beerahee M, Kalberg C, Church A, Kilbride S, Mehta R. Population pharmacokinetics of inhaled umeclidinium and vilanterol in patients with chronic obstructive pulmonary disease. Clinical Pharmacokinetics 2014;53(7):637‐48. [CENTRAL: 994400; CRS: 4900126000016585; EMBASE: 2014444842] - PubMed
-
- NCT01313637. A 24‐week, randomised, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of GSK573719/GW642444 inhalation powder and the individual components delivered once‐daily via a novel dry powder inhaler in subjects with chronic obstructive pulmonary disease. clinicaltrials.gov/show/NCT01313637 (first received 10 March 2011). [CRS: 4900132000005772]
Donohue 2013 {published and unpublished data}
-
- Donohue JF, Maleki‐Yazdi MR, Kilbride S, Mehta R, Kalberg C, Church A. Efficacy and safety of once‐daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respiratory Medicine 2013;107(10):1538‐46. [CENTRAL: 991431; CRS: 4900126000012283; PUBMED: 23830094] - PubMed
-
- Donohue JF, Maleki‐Yazdi MR, Kilbride S, Mehta R, Kalberg C, Church A. Efficacy and safety of once‐daily umeclidinium/vilanterol 62.5/25 mcg in COPD: a randomised, placebo‐controlled, 24‐week study. American Journal of Respiratory and Critical Care Medicine 2013;107(Meeting Abstracts):1538‐46. [CENTRAL: 870702; CRS: 4900100000087847; EMBASE: 2013612450]
-
- GSK573719/GW642444. A 24‐week, randomised, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of GSK573719/GW642444 inhalation powder and the individual components delivered once‐daily via a novel dry powder inhaler in subjects with chronic obstructive pulmonary disease. http://www.gsk‐clinicalstudyregister.com/files/113373/3623/gsk‐113373‐clinical‐study‐re....
-
- Goyal N, Beerahee M, Kalberg C, Church A, Kilbride S, Mehta R. Population pharmacokinetics of inhaled umeclidinium and vilanterol in patients with chronic obstructive pulmonary disease. Clinical Pharmacokinetics 2014;53(7):637‐48. [CENTRAL: 994400; CRS: 4900126000016585; EMBASE: 2014444842] - PubMed
-
- NCT01313650. A 24‐week evaluation of GSK573719/vilanterol (62.5/25mcg) and components in COPD (DB2113373) [A 24‐week, randomised, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of GSK573719/GW642444 inhalation powder and the individual components delivered once‐daily via a novel dry powder inhaler in subjects with chronic obstructive pulmonary disease]. https://clinicaltrials.gov/show/NCT01313650 (first received 10 March 2011). [CRS: 4900132000005786]
Donohue 2014 {published and unpublished data}
-
- DB2113359. A 52 week study to evaluate the safety and tolerability of GSK573719/GW642444 125mcg once‐daily alone and in combination with GW642444 25mcg once‐daily via novel dry powder inhaler (nDPI) in subjects with chronic obstructive pulmonary disease. http://www.gsk‐clinicalstudyregister.com/files/113359/2594/gsk‐113359‐clinical‐study‐re... (first received 27 January 2011).
-
- Donohue J, Niewoehner D, Brooks J, O'Dell D, Church A. Long‐term safety and tolerability of umeclidinium/vilanterol and umeclidinium in COPD [Abstract]. European Respiratory Society Annual Congress; 2013 Sept 7‐11; Barcelona. 2013; Vol. 42, issue Suppl 57:144s [P760]. [CENTRAL: 973473; CRS: 4900126000006692; EMBASE: 71842511]
-
- Donohue JF, Niewoehner D, Brooks J, O'Dell D, Church A. Safety and tolerability of once‐daily umeclidinium/vilanterol 125/25 mcg and umeclidinium 125 mcg in patients with chronic obstructive pulmonary disease: results from a 52‐week, randomised, double‐blind, placebo‐controlled study. Respiratory Research 2014;15:78. [CENTRAL: 998701; CRS: 4900131000000485; EMBASE: 2015832107; PUBMED: 25015176] - PMC - PubMed
-
- NCT01316887. A 52 week study to evaluate the safety and tolerability of GSK573719/GW642444 125mcg once‐daily alone and in combination with GW642444 25mcg once‐daily via novel dry powder inhaler (nDPI) in subjects with chronic obstructive pulmonary disease. 2011, https://clinicaltrials.gov/show/NCT01316887. [CRS: 4900132000005785]
Trivedi 2014 {published and unpublished data}
-
- GSK573719. A12‐week, randomised, double‐blind, placebo‐controlled, parallel‐group study to evaluate the efficacy and safety of GSK573719 delivered once‐daily via a novel dry powder inhaler in subjects with chronic obstructive pulmonary disease. http://www.gsk‐clinicalstudyregister.com/files/115408/2802/gsk‐115408‐clinical‐study‐re... (first received 16 November 2012).
-
- NCT01387230. Evaluate the efficacy and safety of GSK573719 delivered via a novel dry powder inhaler in subjects with COPD [A12‐week, randomised, double‐blind, placebo‐controlled, parallel‐group study to evaluate the efficacy and safety of GSK573719 delivered once‐daily via a novel dry powder inhaler in subjects with chronic obstructive pulmonary disease]. clinicaltrials.gov/show/NCT01387230 (first received 30 June 2011). [CRS: 4900132000005763]
-
- Trivedi R, Richard N, Mehta R, Church A. Efficacy and safety of umeclidinium monotherapy once daily in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A2437. [CENTRAL: 870788; CRS: 4900100000087933]
-
- Trivedi R, Richard N, Mehta R, Church A. Umeclidinium in patients with COPD: a randomised, placebo‐controlled study. European Respiratory Journal 2014;43(1):72‐81. [CENTRAL: 979241; CRS: 4900126000007159; EMBASE: 2014530233; PUBMED: 23949963] - PubMed
References to studies excluded from this review
ACTRN12616001208493 {unpublished data only}
-
- ACTRN12616001208493. Continuous maximal bronchodilatation with UMEC/VI as first line treatment for smokers at risk of developing COPD. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12616001208493 (first received 1 September 2016).
Brealey 2015 {published and unpublished data}
-
- Allen A, Henderson A, Gupta A, Renaux J, Brealey N. Pharmacokinetic (PK) analysis of fluticasone furoate (FF), umeclidinium (UMEC) and vilanterol (VI) following triple therapy in healthy subjects [Abstract]. European Respiratory Journal 2014;44(Suppl 58):P895. [CENTRAL: 1053357; CRS: 4900126000028544; EMBASE: 71849990]
-
- CTT116415. A randomised, double‐blind, single dose, four way cross‐over study to assess the systemic exposure, systemic pharmacodynamics and safety and tolerability of fluticasone furoate, umeclidinium and vilanterol following single inhaled doses of umeclidinium/vilanterol blend + fluticasone furoate, umeclidinium + vilanterol, fluticasone furoate + vilanterol and fluticasone furoate + umeclidinium in healthy subjects. http://www.gsk‐clinicalstudyregister.com/files2/969372f9‐5c53‐4d27‐8aba‐1160dd8c6b08 (first received 17 December 2012).
-
- NCT01691547. A randomised, double‐blind, single dose, four way cross‐over study to assess the systemic exposure, systemic pharmacodynamics and safety and tolerability of fluticasone furoate, umeclidinium and vilanterol following single inhaled doses of umeclidinium/vilanterol blend + fluticasone furoate, umeclidinium + vilanterol, fluticasone furoate + vilanterol and fluticasone furoate + umeclidinium in healthy subjects. clinicaltrials.gov/show/NCT01691547 (first received 20 September 2012).
Brealey 2015a {published and unpublished data}
-
- 200587. An open label, randomised, four‐period crossover, single dose study in healthy volunteers to evaluate the pharmacokinetics of FF/UMEC/VI combination administered at dose levels 100/62.5/25 mcg and 100/125/25 mcg and in comparison with FF/VI (100/25 mcg) and UMEC/VI (62.5/25 mcg). gsk‐clinicalstudyregister.com/files2/gsk‐200587‐clinical‐study‐result‐summar... (first received 15 July 2013).
-
- Henderson A, Allen A, Gupta A, Renaux J, Brealey N. Pharmacokinetic (PK) analysis of fluticasone furoate (FF), umeclidinium (UMEC) and vilanterol (VI) following triple therapy at two UMEC doses in healthy subjects [Abstract]. European Respiratory Journal 2014;44(Suppl 58):P938. [CENTRAL: 1053420; CRS: 4900126000028611; EMBASE: 71850033]
-
- NCT01894386. Pharmacokinetic study in healthy volunteers to characterise the exposure of fluticasone furoate (FF), vilanterol (VI) and umeclidinium (UMEC) at two different doses [An open label, randomised, four‐period crossover, single dose study in healthy volunteers to evaluate the pharmacokinetics of FF/UMEC/VI combination administered at dose levels 100/62.5/25 mcg and 100/125/25 mcg and in comparison with FF/VI (100/25 mcg) and UMEC/VI (62.5/25 mcg)]. clinicaltrials.gov/show/NCT01894386 (first received 3 July 2013). [CRS: 4900132000005769]
Cahn 2013 {published and unpublished data}
-
- AC4110106. A single centre, randomised, double‐blind, dose ascending, placebo‐controlled study, in two parts, to evaluate the safety, tolerability and pharmacokinetics of escalating single and repeat inhaled doses of GSK573719 and placebo formulated with the excipient magnesium stearate, in healthy subjects and in a healthy population of cytochrome P450 isoenzyme 2D6 poor metabolisers. gsk‐clinicalstudyregister.com/files/110106/5490/gsk‐110106‐clinical‐study‐re... (first received 12 March 2009).
-
- Cahn A, Mehta R, Preece A, Blowers J. Safety, tolerability and pharmacokinetics (PK) of once‐daily GSK573719 in healthy adults lacking CYP2D6 activity [Abstract]. American Journal of Respiratory and Critical Care Medicine. 2012; Vol. 185, issue Meeting Abstracts:A2918. [CENTRAL: 834268; CRS: 4900100000060536]
-
- Cahn A, Mehta R, Preece A, Blowers J, Donald A. Safety, tolerability and pharmacokinetics and pharmacodynamics of inhaled once‐daily umeclidinium in healthy adults deficient in CYP2D6 activity: a double‐blind, randomised clinical trial. Clinical Drug Investigation 2013;33(9):653‐64. [PUBMED: 23881566] - PubMed
-
- NCT00803673. A healthy volunteer study with inhaled GSK573719 and placebo [A single centre, randomised, double‐blind, dose ascending, placebo‐controlled study, in two parts, to evaluate the safety, tolerability and pharmacokinetics of escalating single and repeat inhaled doses of GSK573719 and placebo formulated with the excipient magnesium stearate, in healthy subjects]. https://clinicaltrials.gov/ct2/show/NCT00803673.
Cahn 2013a {published and unpublished data}
-
- AC4105209. A randomised double‐blind placebo‐controlled crossover dose escalation study to examine the safety tolerability pharmacodynamics and pharmacokinetics of inhaled doses of GSK233719 in healthy normal volunteers (single and repeat dosing) and in healthy CYP2D6 poor metaboliser volunteers (single or repeat dosing). http://www.gsk‐clinicalstudyregister.com/files/105209/3754/gsk‐105209‐clinical‐study‐re... (first received 5 October 2010).
-
- AC4106889. A single‐centre, randomised, double‐blind, placebo‐controlled, dose‐ascending, 3‐cohort parallel‐group study to evaluate the safety, tolerability, pharmacodynamics and pharmacokinetics of GSK573719 administered as single doses (750 μg and 1000 μg) and repeat doses over 14 days (250 μg–1000 μg once‐daily) of GSK573719 in healthy male and female subjects.. http://www.gsk‐clinicalstudyregister.com/files/AC4106889/4083/gsk‐ac4106889‐clinical‐st... (first received 13 September 2012).
-
- Cahn A, Lovick R, Newlands A, Deans A, Pouliquen I, Preece A, et al. Safety, tolerability, pharmacodynamics (PD) and pharmacokinetics (PK) of GSK573719 inhalation powder in healthy subjects [Abstract]. European Respiratory Society 21st Annual Congress; 2011 Sep 24‐28; Amsterdam. 2011; Vol. 38, issue 55:723s [P3971]. [CENTRAL: 833568; CRS: 4900100000054076]
-
- Cahn A, Tal‐Singer R, Pouliquen IJ, Mehta R, Preece A, Hardes K, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of single and repeat inhaled doses of umeclidinium in healthy subjects: two randomised studies. Clinical Drug Investigation 2013;33(7):477‐88. [PUBMED: 23784369] - PubMed
-
- Mehta R, Hardes K, Cahn A, Newlands A, Donald A, Preece A, et al. Safety, tolerability and pharmacokinetics (PK) of repeated doses of GSK573719 inhalation powder, a new long‐acting muscarinic antagonist, in healthy adults [Abstract]. European Respiratory Society 21st Annual Congress; 2011 Sep 24‐28; Amsterdam. 2011; Vol. 38, issue 55:723s [P3972]. [CENTRAL: 833569; CRS: 4900100000054077]
Church 2014 {published and unpublished data}
-
- AC4115321. A randomised, double blind, placebo controlled, incomplete block, crossover, dose ranging study to evaluate the dose response of GSK573719 administered once or twice daily over 7 days in patients with chronic obstructive pulmonary disease (COPD). http://www.gsk‐clinicalstudyregister.com/files/115321/3471/gsk‐115321‐clinical‐study‐re... (first received 15 May 2012).
-
- AC4116689. An integrated review to evaluate dose response of umeclidinium (GSK573719) administered once or twice daily in subjects with COPD. www.gsk‐clinicalstudyregister.com/files/116689/3942/gsk‐116689‐clinical‐study‐re... (first received 20 June 2012).
-
- Church A, Beerahee M, Brooks J, Mehta R, Shah P. Umeclidinium (GSK573719) dose response and dosing interval in COPD [Abstract]. European Respiratory Journal 2012;40(Suppl 56):377s [P2121]. [CENTRAL: 839340; CRS: 4900100000067947; EMBASE: 71926592]
-
- Church A, Kalberg C, Shah P, Beerahee M, Donohue J. An analysis of the dose response of umeclidinium (GSK573719) administered once or twice daily in patients with COPD. Chest 2012;142(4):Suppl 1. [CENTRAL: 862206; CRS: 4900100000079532; EMBASE: 71073009]
Decramer 2013 {published and unpublished data}
-
- AC4113589. A randomised, double‐blind, parallel‐group, placebo‐controlled study to evaluate the efficacy and safety of GSK573719 delivered once‐daily over 28 days in subjects with COPD. http://www.gsk‐clinicalstudyregister.com/files/113589/4085/gsk‐113589‐clinical‐study‐re... (first received 12 November 2015).
-
- Decramer M, Maltais F, Feldman G, Brooks J, Harris S, Mehta R, et al. Bronchodilation of umeclidinium, a new long‐acting muscarinic antagonist, in COPD patients. Respiratory Physiology and Neurobiology 2013;185(2):393‐9. [CENTRAL: 839974; CRS: 4900100000070752; EMBASE: 2013014584; PUBMED: 23026438] - PubMed
-
- Decramer M, Maltais F, Feldman G, Brooks J, Willits L, Harris S, et al. Dose‐related efficacy of GSK573719, a new long‐acting muscarinic receptor antagonist (LAMA) offering sustained 24‐hour bronchodilation, in COPD [Abstract]. European Respiratory Society 21st Annual Congress; 2011 Sep 24‐28; Amsterdam. 2011; Vol. 38, issue 55:150s [P878]. [CENTRAL: 833478; CRS: 4900100000053983]
-
- NCT01030965. A 28‐day repeat dose study of GSK573719 [A randomised, double‐blind, parallel‐group, placebo‐controlled study to evaluate the efficacy and safety of GSK573719 delivered once‐daily over 28 days in subjects with COPD]. https://clinicaltrials.gov/show/NCT01030965 (first received 10 December 2009). [CRS: 4900132000005799]
Decramer 2014 {published and unpublished data}
-
- Anzueto A, Decramer M, Kaelin T, Richard N, Tabberer M, Harris S, et al. The efficacy and safety of umeclidinium/vilanterol compared with tiotropium or vilanterol over 24 weeks in subjects with COPD. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A4268. [CENTRAL: 870666; CRS: 4900100000087810]
-
- DB2113360. A multicentre trial comparing the efficacy and safety of GSK573719/GW642444 with GW642444 and with tiotropium over 24 weeks in subjects with COPD. http://www.gsk‐clinicalstudyregister.com/files/113360/2504/gsk‐113360‐clinical‐study‐re... (first received 21 March 2011).
-
- Decramer M, Anzueto A, Kerwin E, Kaelin T, Richard N, Crater G, et al. Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trials. Lancet Respiratory Medicine 2014;2:472‐86. [PUBMED: 24835833] - PubMed
-
- NCT01316900. A 24‐week trial comparing GSK573719/GW642444 with GW642444 and with tiotropium in chronic obstructive pulmonary disease [A multicentre trial comparing the efficacy and safety of GSK573719/GW642444 with GW642444 and with tiotropium over 24 weeks in subjects with COPD]. https://clinicaltrials.gov/show/NCT01316900 (first received 15 March 2011). [CRS: 4900132000005775]
Decramer 2014a {published and unpublished data}
-
- DB2113374. A multi‐centre trial comparing the efficacy and safety of GSK573719/GW642444 with GSK573719 and with tiotropium over 24 weeks in subjects with COPD. http://www.gsk‐clinicalstudyregister.com/files/113374/2588/gsk‐113374‐clinical‐study‐re... (first received 21 March 2011).
-
- Decramer M, Anzueto A, Kerwin E, Kaelin T, Richard N, Crater G, et al. Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trials. Lancet Respiratory Medicine 2014;2:472‐86. [PUBMED: 24835833] - PubMed
-
- Decramer M, Anzueto A, Kerwin E, Richard N, Crater G, Tabberer M, et al. Efficacy and safety of umeclidinium/vilanterol compared with umeclidinium or tiotropium in COPD [Abstract]. European Respiratory Society 23rd Annual Congress; 2013 Sep 7‐11; Barcelona. 2013; Vol. 42, issue Suppl 57:751s [P3640]. [CENTRAL: 973468; CRS: 4900126000006687; EMBASE: 71842563]
-
- NCT01316913. A multi‐centre trial comparing the efficacy and safety of GSK573719/GW642444 with GSK573719 and with tiotropium over 24 weeks in subjects with COPD. https://clinicaltrials.gov/show/NCT01316913 (first received 15 March 2011). [CRS: 4900132000005801]
Donohue 2012 {published and unpublished data}
-
- AC4113073. A randomised, double‐blind, placebo‐controlled, 3‐way cross‐over study to evaluate the safety, efficacy, and pharmacokinetics of GSK573719 administered once‐ and twice‐daily in subjects with COPD. http://www.gsk‐clinicalstudyregister.com/files/113073/3833/gsk‐113073‐clinical‐study‐re... (first received 28 June 2012).
-
- Church A, Kalberg C, Shah P, Beerahee M, Donohue J. An analysis of the dose response of umeclidinium (GSK573719) administered once or twice daily in patients with COPD. Chest 2012;142(4):Suppl 1. [CENTRAL: 862206; CRS: 4900100000079532; EMBASE: 71073009]
-
- Donohue J, Anzueto A, Brooks J, Mehta R, Kalberg C, Crater G. Dose‐related efficacy of GSK573719: a long‐acting muscarinic receptor antagonist (LAMA) with sustained 24‐hour activity in COPD [Abstract]. Chest. 2011; Vol. 140, issue 4:1043A. [CENTRAL: 833269; CRS: 4900100000034631; EMBASE: 70635385]
-
- Donohue JF, Anzueto A, Brooks J, Mehta R, Kalberg C, Crater G. A randomised, double‐blind dose‐ranging study of the novel LAMA GSK573719 in patients with COPD. Respiratory Medicine 2012;106(7):970‐9. [CENTRAL: 834183; CRS: 4900100000058425; EMBASE: 2012283145; PUBMED: 22498110] - PubMed
-
- Donohue JF, Kalberg C, Shah P, Beerahee M, Mehta R, Gunawan R, et al. Dose response of umeclidinium administered once or twice daily in patients with COPD: a pooled analysis of two randomised, double‐blind, placebo‐controlled studies. Journal of Clinical Pharmacology 2014;54(11):1214‐20. [CENTRAL: 1068117; CRS: 4900132000000599; EMBASE: 2015884254] - PubMed
Donohue 2015 {published and unpublished data}
-
- DB2114930. A randomised, multi‐centre, double‐blind, double‐dummy, parallel group study to evaluate the efficacy and safety of umeclidinium/vilanterol compared with fluticasone propionate/salmeterol over 12 weeks in subjects with COPD. http://www.gsk‐clinicalstudyregister.com/files/114930/4353/gsk‐114930‐clinical‐study‐re... (first received 24 March 2014).
-
- Donohue J, Worsley S, Zhu C‐Q, Hardaker L, Church A. Efficacy and safety of umeclidinium/vilanterol (UMEC/VI) once daily (OD) vs fluticasone/salmeterol combination (FSC) twice daily (BD) in patients with moderate‐to‐severe COPD and infrequent COPD exacerbations. Chest 2014;146:4. [CENTRAL: 1051011; CRS: 4900126000026313; EMBASE: 71780205]
-
- Donohue JF, Worsley S, Zhu C‐Q, Hardaker L, Church A. Improvements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate‐to‐severe COPD and infrequent exacerbations. Respiratory Medicine 2015;109(7):870‐81. [CENTRAL: 1072991; CRS: 4900132000002651; EMBASE: 2015066377; PUBMED: 26006754] - PubMed
-
- EUCTR2012‐000525‐45‐GR. Study to compare umeclidinium/vilanterol compared with fluticasone propionate/salmeterol in COPD [DB2114930: a randomised, multi‐centre, double‐blind, double‐dummy, parallel group study to evaluate the efficacy and safety of umeclidinium/vilanterol compared with fluticasone propionate/salmeterol over 12 weeks in subjects with COPD]. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2012‐000525‐45‐GR (first received 11 December 2012).
-
- NCT01817764. A study to compare the efficacy and safety of umeclidinium/vilanterol and fluticasone propionate/salmeterol in subjects with chronic obstructive pulmonary disease (COPD) [DB2114930: a randomised, multi‐centre, double‐blind, double‐dummy, parallel group study to evaluate the efficacy and safety of umeclidinium/vilanterol compared with fluticasone propionate/salmeterol over 12 weeks in subjects with COPD]. clinicaltrials.gov/show/NCT01817764 (first received 21 March 2013). [CRS: 4900132000005774]
Donohue 2015a {published and unpublished data}
-
- DB2114951. A randomised, multi‐centre, double‐blind, double‐dummy, parallel group study to evaluate the efficacy umeclidinium/vilanterol compared with fluticasone propionate/salmeterol over 12 weeks in subjects with COPD. http://www.gsk‐clinicalstudyregister.com/files/114951/4500/gsk‐114951‐clinical‐study‐re... (first received 13 June 2013).
-
- Donohue J, Worsley S, Zhu C‐Q, Hardaker L, Church A. Efficacy and safety of umeclidinium/vilanterol (UMEC/VI) once daily (OD) vs fluticasone/salmeterol combination (FSC) twice daily (BD) in patients with moderate‐to‐severe COPD and infrequent COPD exacerbations. Chest 2014;146(4_MeetingAbstracts):73A. [CENTRAL: 1051011; CRS: 4900126000026313; EMBASE: 71780205]
-
- Donohue JF, Worsley S, Zhu C‐Q, Hardaker L, Church A. Improvements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate‐to‐severe COPD and infrequent exacerbations. Respiratory Medicine 2015;109(7):870‐81. [CENTRAL: 1072991; CRS: 4900132000002651; EMBASE: 2015066377; PUBMED: 26006754] - PubMed
-
- EUCTR2012‐002156‐16‐RO. A randomised, multi‐centre, double‐blind, double dummy, parallel group study to evaluate the efficacy and safety of umeclidinium/vilanterol compared with fluticasone propionate/salmeterol over 12 weeks in subjects with COPD. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2012‐002156‐16‐RO (first received 28 July 2014).
-
- NCT01879410. A study to compare the efficacy and safety of umeclidinium/vilanterol with fluticasone propionate/salmeterol in subjects with chronic obstructive pulmonary disease (COPD) [DB2114951: a randomised, multi‐centre, double‐blind, double‐dummy, parallel group study to evaluate the efficacy umeclidinium/vilanterol compared with fluticasone propionate/salmeterol over 12 weeks in subjects with COPD]. https://clinicaltrials.gov/show/NCT01879410 (first received 13 June 2013). [CRS: 4900132000005792]
Donohue 2016 {published and unpublished data}
-
- DB2116132. A randomised, double‐blind, 3‐way, cross‐over study to evaluate lung function response after treatment with umeclidinium 62.5 mcg, vilanterol 25 mcg, and umeclidinium/vilanterol 62.5/25 mcg once‐daily in subjects with chronic obstructive pulmonary disease (COPD). http://www.gsk‐clinicalstudyregister.com/files2/2aab8794‐387d‐49fd‐ab26‐63529c6c7ae3 (first received 5 February 2013).
-
- Donohue JF, Singh D, Munzu C, Kilbride S, Church A. Magnitude of umeclidinium/vilanterol lung function effect depends on monotherapy responses: results from two randomised controlled trials. Respiratory Medicine 2016;112:65‐74. [CENTRAL: 1135183; CRS: 4900132000015040; EMBASE: 20160087204; PUBMED: 26797016] - PubMed
-
- EUCTR2011‐005913‐35‐SK. A randomised, double‐blind, 3‐way, cross‐over study to evaluate lung function response after treatment with umeclidinium 62.5 mcg, vilanterol 25 mcg, and umeclidinium/vilanterol 62.5/25 mcg once‐daily in subjects with chronic obstructive pulmonary disease (COPD). http://apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2011‐005913‐35‐SK (first received 9 March 2016).
-
- NCT02014480. A cross‐over study to evaluate lung function response after treatment with umeclidinium (UMEC) 62.5 micrograms (mcg), vilanterol (VI) 25 mcg, and umeclidinium/vilanterol (UMEC/VI) 62.5/25 mcg once‐daily in subjects with chronic obstructive pulmonary disease (COPD) [A randomised, double‐blind, 3‐way, cross‐over study to evaluate lung function response after treatment with umeclidinium 62.5 mcg, vilanterol 25 mcg, and umeclidinium/vilanterol 62.5/25 mcg once‐daily in subjects with chronic obstructive pulmonary disease (COPD)]. clinicaltrials.gov/show/NCT02014480 (first received 12 December 2013). [CRS: 4900132000005761]
Donohue 2016a {published and unpublished data}
-
- DB2116133. A randomised, double‐blind, 3‐way, cross‐over study to evaluate lung function response after treatment with umeclidinium 62.5 mcg, vilanterol 25 mcg, and umeclidinium/vilanterol 62.5/25 mcg once‐daily in subjects with chronic obstructive pulmonary disease (COPD). http://www.gsk‐clinicalstudyregister.com/files2/a4eb7507‐0808‐4196‐b85d‐70c63e287917 (first received 19 October 2012).
-
- Donohue JF, Singh D, Munzu C, Kilbride S, Church A. Magnitude of umeclidinium/vilanterol lung function effect depends on monotherapy responses: results from two randomised controlled trials. Respiratory Medicine 2016;112:65‐74. [CENTRAL: 1135183; CRS: 4900132000015040; EMBASE: 20160087204; PUBMED: 26797016] - PubMed
-
- EUCTR2011‐005914‐12‐DE. A randomised, double‐blind, 3‐way, cross‐over study to evaluate lung function response after treatment with umeclidinium 62.5 mcg, vilanterol 25 mcg, and umeclidinium/vilanterol 62.5/25 mcg once‐daily in subjects with chronic obstructive pulmonary disease (COPD). apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2011‐005914‐12‐DE (first received 5 June 2012).
-
- NCT01716520. Cross‐over study in subjects with COPD, evaluating lung function response after treatment with once daily umeclidinium 62.5mcg, vilanterol 25mcg, and umeclidinium/vilanterol 62.5/25mcg [A randomised, double‐blind, 3‐way, cross‐over study to evaluate lung function response after treatment with umeclidinium 62.5 mcg, vilanterol 25 mcg, and umeclidinium/vilanterol 62.5/25 mcg once‐daily in subjects with chronic obstructive pulmonary disease (COPD)]. https://clinicaltrials.gov/show/NCT01716520 (first received 11 October 2012). [CRS: 4900132000005777]
Feldman 2012 {published and unpublished data}
-
- DB2113120. Safety, tolerability, pharmacokinetics and pharmacodynamics of the combination of GSK573719 and GW642444 in subjects with COPD. gsk‐clinicalstudyregister.com/files/113120/1545/gsk‐113120‐clinical‐study‐re... (first received 18 August 2010).
-
- Feldman G, Walker RR, Brooks J, Mehta R, Crater G. A 28‐day safety and tolerability of umeclidinium in combination with vilanterol in COPD: a randomised placebo‐controlled trial. Pulmonary Pharmacology and Therapeutics 2012;25(6):465‐71. [CENTRAL: 839948; CRS: 4900100000070656; EMBASE: 2012699622; PUBMED: 22955035] - PubMed
-
- Feldman G, Walker RR, Brooks J, Mehta R, Crater G. Safety and tolerability of the GSK573719/vilanterol combination In patients with COPD [Abstract]. American Journal of Respiratory and Critical Care Medicine. 2012; Vol. 185:A2938. [CENTRAL: 834400; CRS: 4900100000060668]
-
- NCT01039675. Safety, tolerability, pharmacokinetics and pharmacodynamics of the combination of GSK573719 and GW642444 in subjects with COPD. clinicaltrials.gov/show/NCT01039675 (first received 23 December 2009). [CRS: 4900132000005766]
Feldman 2016 {published and unpublished data}
-
- 201316. A randomised, blinded, double‐dummy, parallel‐group study to evaluate the efficacy and safety of umeclidinium (UMEC) 62.5 mcg compared with tiotropium 18 mcg in subjects with chronic obstructive pulmonary disease (COPD). http://www.gsk‐clinicalstudyregister.com/files2/gsk‐201316‐clinical‐study‐result‐summar... (first received 30 September 2014).
-
- EUCTR2014‐000884‐42‐DE. A randomised, blinded, double‐dummy, parallel‐group study to evaluate the efficacy and safety of umeclidinium (UMEC) 62.5 mcg compared with tiotropium 18 mcg in subjects with chronic obstructive pulmonary disease (COPD). http://apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2014‐000884‐42‐DE (first received 13 June 2014).
-
- Feldman G, Maltais F, Khindri S, Vahdati‐Bolouri M, Church A, Fahy WA, et al. A randomised, blinded study to evaluate the efficacy and safety of umeclidinium 62.5 µg compared with tiotropium 18 µg in patients with COPD. International Journal of Chronic Obstructive Pulmonary Disease 2016;11(1):719‐30. [CENTRAL: 1152854; CRS: 4900132000019445; EMBASE: 20160300103; PUBMED: 27103795] - PMC - PubMed
-
- NCT02207829. A 12‐week study to evaluate the efficacy and safety of umeclidinium compared with tiotropium in subjects with chronic obstructive pulmonary disease [A randomised, blinded, double‐dummy, parallel‐group study to evaluate the efficacy and safety of umeclidinium (UMEC) 62.5 mcg compared with tiotropium 18 mcg in subjects with chronic obstructive pulmonary disease (COPD)]. https://clinicaltrials.gov/show/NCT02207829 (first received 31 July 2014). [CRS: 4900132000005800]
Hu 2015 {published and unpublished data}
-
- DB2115380. A randomised, open label, 3 crossover, balanced incomplete block study to evaluate the pharmacokinetics of umeclidinium bromide and vilanterol trifenatate as monotherapies and concurrently in healthy Chinese subjects.. http://www.gsk‐clinicalstudyregister.com/files/115380/4571/gsk‐115380‐clinical‐study‐re... (first received 18 August 2010).
-
- NCT01899638. Pharmacokinetics of umeclidinium and vilanterol in healthy Chinese, a randomised, open label, 3 crossover study. clinicaltrials.gov/ct2/show/NCT01899638 (first received 30 May 2013).
Kalberg 2016 {published and unpublished data}
-
- DB2116961. A multicentre, randomised, blinded, parallel group study to compare UMEC/VI (umeclidinium/vilanterol) in a fixed dose combination with indacaterol plus tiotropium in symptomatic subjects with moderate to very severe COPD. http://www.gsk‐clinicalstudyregister.com/files2/gsk‐116961‐Clinical‐Study‐Result‐Summar... (first received 15 October 2014).
-
- EUCTR2013‐001827‐38‐DE. Study DB2116961, a multicentre, randomised, blinded, parallel group study to compare UMEC/VI (umeclidinium/vilanterol) in a fixed dose combination with indacaterol plus tiotropium in symptomatic subjects with moderate to very severe COPD. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013‐001827‐38‐DE (first received 19 May 2014).
-
- Kalberg C, O'Dell D, Galkin D, Newlands A, Fahy WA. Dual bronchodilator therapy with umeclidinium/vilanterol versus tiotropium plus indacaterol in chronic obstructive pulmonary disease: a randomised controlled trial. Drugs in R&D 2016;16(2):217‐27. [CENTRAL: 1139853; CRS: 4900132000017651; EMBASE: 20160259248; PUBMED: 27028749] - PMC - PubMed
-
- NCT02257385. Comparative study of umeclidinium/vilanterol (UMEC/VI) in a fixed dose combination with indacaterol plus tiotropium. clinicaltrials.gov/ct2/show/NCT02257385 (first received 2 October 2014).
-
- PER‐018‐14. Study DB2116961, a multicentre, randomised, blinded, double‐dummy, parallel group study to compare UMEC/VI (umeclidinium/vilanterol) in a fixed dose combination with indacaterol plus tiotropium in symptomatic subjects with moderate to very severe COPD. apps.who.int/trialsearch/Trial2.aspx?TrialID=PER‐018‐14 (first received 12 August 2014).
Kelleher 2011 {published and unpublished data}
-
- AC4105211. A randomised, double‐blind, placebo‐controlled, dose ascending, 2‐cohort, parallel group study to examine the safety, tolerability and pharmacokinetics of once‐daily inhaled doses of GSK573719 formulated with the excipient magnesium stearate in COPD subjects for 7 days. gsk‐clinicalstudyregister.com/files/105211/3794/gsk‐105211‐clinical‐study‐re... (first received 20 January 2010).
-
- Kelleher D, Preece A, Mehta R, Donald A, Hardes K, Cahn A, et al. Phase II study of once‐daily GSK573719 inhalation powder, a new long‐acting muscarinic antagonist, in patients with chronic obstructive pulmonary disease (COPD) [Abstract]. European Respiratory Society 21st Annual Congress; 2011 Sep 24‐28; Amsterdam. 2011; Vol. 38, issue 55:140s [P834]. [CENTRAL: 833467; CRS: 4900100000053971]
-
- NCT00732472. A study to assess the safety and tolerability of once daily inhaled doses of GSK573719 made with magnesium stearate in subjects with chronic obstructive pulmonary disease(COPD) for 7 Days [A randomised, double‐blind, placebo‐controlled, dose ascending, 2‐cohort, parallel group study to examine the safety, tolerability and pharmacokinetics of once‐daily inhaled doses of GSK573719 formulated with the excipient magnesium stearate in COPD subjects for 7 days]. clinicaltrials.gov/show/NCT00732472 (first received 11 August 2008). [CRS: 4900132000005765]
-
- Tal‐Singer R, Cahn A, Mehta R, Preece A, Crater G, Kelleher D, et al. Initial assessment of single and repeat doses of inhaled umeclidinium in patients with chronic obstructive pulmonary disease: two randomised studies. European Journal of Pharmacology 2013;701(1‐3):40‐8. [CENTRAL: 853353; CRS: 4900100000074852; EMBASE: 2013146506; PUBMED: 23276660] - PubMed
Kelleher 2012 {published and unpublished data}
-
- DB2113208. A single centre, randomised, placebo‐controlled, four‐way cross‐over study to assess the safety, tolerability, pharmacodynamics and pharmacokinetics of single inhaled doses of GSK573719 and GW642444 as monotherapies and concurrently in healthy Japanese subjects. gsk‐clinicalstudyregister.com/files/113208/4904/gsk‐113208‐clinical‐study‐re... (first received 3 March 2010).
-
- Kelleher D, Mehta R, Jean‐Francois B, Preece A, Blowers J, Crater G. Safety, tolerability, pharmacodynamics (PD) and pharmacokinetics (PK) of single inhaled doses of GSK573719 and vilanterol (VI) when administered separately and in combination to healthy adult Japanese subjects [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185:A2916. [CENTRAL: 834258; CRS: 4900100000060526]
-
- Kelleher DL, Mehta RS, Jean‐Francois BM, Preece AF, Blowers J, Crater GD, et al. Safety, tolerability, pharmacodynamics and pharmacokinetics of umeclidinium and vilanterol alone and in combination: a randomised crossover trial. PloS One 2012;7(12):e50716. [CENTRAL: 839982; CRS: 4900100000070763; EMBASE: 2012742045; PUBMED: 23284643] - PMC - PubMed
-
- NCT00976144. Safety, tolerability, pharmacokinetic and pharmacodynamic effects of GSK573719 (LAMA) and GW642444 (LABA) administered individually and concurrently in healthy Japanese subjects (DB2113208) [A single centre, randomised, placebo‐controlled, four‐way cross‐over study to assess the safety, tolerability, pharmacodynamics and pharmacokinetics of single inhaled doses of GSK573719 and GW642444 as monotherapies and concurrently in healthy Japanese subjects]. clinicaltrials.gov/show/NCT00976144 (first received 3 September 2009). [CRS: 4900132000005802]
Kelleher 2012a {published and unpublished data}
-
- AC4112014. An open‐label, two period study to determine the excretion balance and pharmacokinetics of 14C‐GSK573719, administered as single dose of an oral solution and an intravenous infusion, to healthy male adults. gsk‐clinicalstudyregister.com/files/112014/3810/gsk‐112014‐clinical‐study‐re... (first received 13 September 2012).
-
- Kelleher D, Hughes S, Mehta R, Tombs L, Kelly K, Church A. Absorption, distribution, metabolism, and elimination (ADME) of umeclidinium (UMEC) in healthy adults. European Respiratory Journal 2012;40(Suppl 56):384s [P2153].
-
- NCT01362257. A study to determine the excretion balance and pharmacokinetics of 14C‐GSK573719. clinicaltrials.gov/ct2/show/NCT01362257 (first received 26 May 2011).
Kelleher 2014 {published and unpublished data}
-
- DB2114635. A randomised, placebo‐controlled, incomplete block, four period crossover, repeat dose study to evaluate the effect of the inhaled GSK573719/vilanterol combination and GSK573719 monotherapy on electrocardiographic parameters, with moxifloxacin as a positive control, in healthy subjects. gsk‐clinicalstudyregister.com/files/114635/5724/gsk‐114635‐clinical‐study‐re... (first received 17 October 2012).
-
- Kelleher D, Tombs L, Crater G, Preece A, Brealey N, Mehta R. A placebo‐ and moxifloxacin‐controlled thorough QT study of umeclidinium monotherapy and umeclidinium/vilanterol combination in healthy subjects. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A1487. [CENTRAL: 870724; CRS: 4900100000087869]
-
- Kelleher D, Tombs L, Preece A, Brealey N, Mehta R. A randomised, placebo‐ and moxifloxacin‐controlled thorough QT study of umeclidinium monotherapy and umeclidinium/vilanterol combination in healthy subjects. Pulmonary Pharmacology and Therapeutics 2014;29(1):49‐57. [PUBMED: 25020273] - PubMed
-
- NCT01521377. A randomised, placebo‐controlled, incomplete block, four period crossover, repeat dose study to evaluate the effect of the inhaled GSK573719/vilanterol combination and GSK573719 monotherapy on electrocardiographic parameters, with moxifloxacin as a positive control, in healthy subjects. clinicaltrials.gov/show/NCT01521377 (first received 26 January 2012). [CRS: 4900132000005794]
Kerwin 2017 {published and unpublished data}
-
- DB2116960. A randomised, double‐dummy, parallel group, multicentre trial comparing the efficacy and safety of UMEC/VI (a fixed combination of umeclidinium and vilanterol) with tiotropium in subjects with COPD who continue to have symptoms on tiotropium. gsk‐clinicalstudyregister.com/files2/gsk‐116960‐Clinical‐Study‐Result‐Summar... (first received 15 September 2004).
-
- EUCTR2012‐005007‐41‐SE. A randomised, double‐blind, double‐dummy, parallel group study comparing UMEC/VI (a fixed combination of umeclidinium and vilanterol) with tiotropium in COPD subjects who continue to have symptoms on tiotropium. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2012‐005007‐41‐SE (first received 19 April 2013).
-
- Kerwin E, Kalberg CJ, Galkin D, Zhu C‐Q, Church A, Fahy W. UMEC/VI as step‐up therapy from tiotropium in moderate symptomatic COPD: a randomised, 12‐week study. American Journal of Respiratory and Critical Care Medicine 2016;193(Meeting Abstracts):A6797. [CENTRAL: 1261962; CRS: 4900132000041861]
-
- Kerwin EM, Kalberg CJ, Galkin DV, Zhu CQ, Church A, Riley JH, et al. Umeclidinium/vilanterol as step‐up therapy from tiotropium in patients with moderate COPD: a randomised, parallel‐group, 12‐week study. International Journal of Chronic Obstructive Pulmonary Disease 2017;12:745‐55. [CRS: 4900132000046361; EMBASE: 614653439; PUBMED: 28280319] - PMC - PubMed
-
- NCT01899742. DB2116960. A randomised, double‐dummy, parallel group, multicentre trial comparing the efficacy and safety of UMEC/VI (a fixed combination of umeclidinium and vilanterol) with tiotropium in subjects with COPD who continue to have symptoms on tiotropium. clinicaltrials.gov/show/NCT01899742 (first received 11 July 2013). [CRS: 4900132000005791]
Lomas 2016 {published and unpublished data}
-
- CTT116853. A phase III, 24 week, randomised, double‐blind, double‐dummy, parallel group study (with an extension to 52 weeks in a subset of subjects) comparing the efficacy, safety and tolerability of the fixed dose triple combination FF/UMEC/VI administered once‐daily in the morning via a dry powder inhaler with budesonide/formoterol 400mcg/12mcg administered twice‐daily via a reservoir inhaler in subjects with chronic obstructive pulmonary disease. gsk‐clinicalstudyregister.com/study/116853#ps (first received January 2015).
-
- EUCTR2013‐003073‐10‐IT. A phase III, 24 week, randomised, double‐blind, double‐dummy, parallel group study (with an extension to 52 weeks in a subset of subjects) comparing the efficacy, safety and tolerability of the fixed dose triple combination FF/UMEC/VI administered once‐daily in the morning via a dry powder inhaler with budesonide/formoterol 400mcg/12mcg administered twice‐daily via a reservoir inhaler in subjects with chronic obstructive pulmonary disease. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013‐003073‐10‐IT (first received 13 June 2014).
-
- Lomas D, Lipson D, Barnacle H, Birk R, Brealey N, Zhu C‐Q, et al. Single inhaler triple therapy (ICS/LAMA/LABA) in patients with advanced COPD: results of the FULFIL trial. European Respiratory Journal 2016;48(Suppl 60):PA4629. [CRS: 4900132000046884]
-
- NCT02345161. A comparison study between the fixed dose triple combination of fluticasone furoate/umeclidinium/vilanterol trifenatate (FF/UMEC/VI) with budesonide/formoterol in subjects with chronic obstructive pulmonary disease (COPD) [A phase III, 24 week, randomised, double‐blind, double‐dummy, parallel group study (with an extension to 52 weeks in a subset of subjects) comparing the efficacy, safety and tolerability of the fixed dose triple combination FF/UMEC/VI administered once‐daily in the morning via a dry powder inhaler with budesonide/formoterol 400mcg/12mcg administered twice‐daily via a reservoir inhaler in subjects with chronic obstructive pulmonary disease]. clinicaltrials.gov/show/NCT02345161 (first received 19 January 2015). [CRS: 4900132000005793]
Maleki‐Yazdi 2014 {published and unpublished data}
-
- EUCTR2012‐003973‐24‐HU. A multicentre, trial comparing the efficacy and safety of umeclidinium/vilanterol 62.5/25 mcg once daily with tiotropium 18 mcg once daily over 24 weeks in subjects with chronic obstructive pulmonary disease (COPD). apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2012‐003973‐24‐HU (first received 11 December 2012).
-
- Maleki‐Yazdi MR, Kaelin T, Richard N, Zvarich M, Church A. Efficacy and safety of umeclidinium/vilanterol 62.5/25 mcg and tiotropium 18 mcg in chronic obstructive pulmonary disease: results of a 24‐week, randomised, controlled trial. Respiratory Medicine 2014;108(12):1752‐60. [CENTRAL: 1020060; CRS: 4900126000022525; EMBASE: 2015927031; PUBMED: 25458157] - PubMed
-
- NCT01777334. A multicentre, trial comparing the efficacy and safety of umeclidinium/vilanterol 62.5/25 mcg once daily with tiotropium 18 mcg once daily over 24 weeks in subjects with chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/show/NCT01777334 (first received 24 January 2013). [CRS: 4900132000005804]
-
- Singh D, Maleki‐Yazdi MR, Tombs L, Iqbal A, Fahy WA, Naya I. Prevention of clinically important deteriorations in COPD with umeclidinium/vilanterol. International Journal of Chronic Obstructive Pulmonary Disease 2016;11(1):1413‐24. [CRS: 4900132000026674; EMBASE: 20160493257; PUBMED: 27445468] - PMC - PubMed
-
- ZEP117115. A multicentre, trial comparing the efficacy and safety of umeclidinium/vilanterol 62.5/25 mcg once daily with tiotropium 18 mcg once daily over 24 weeks in subjects with chronic obstructive pulmonary disease (COPD). gsk‐clinicalstudyregister.com/files2/117115‐Clinical‐Study‐Result‐Summary.pdf (first received 23 January 2013).
Maltais 2014 {published and unpublished data}
-
- DB2114417. An exercise endurance study to evaluate the effects of treatment of chronic obstructive pulmonary disease (COPD) patients with a dual bronchodilator: GSK573719/GW642444. Study A. gsk‐clinicalstudyregister.com/files/114417/2631/gsk‐114417‐clinical‐study‐re... (first received 16 March 2011).
-
- Maltais F, Singh S, Donald A, Church A, Crater G, Goh A, et al. Effects of a combination of vilanterol and umeclidinium on exercise endurance in subjects with COPD: two randomised clinical trials [Abstract]. European Respiratory Society 23rd Annual Congress; 2013 Sep 7‐11; Barcelona. 2013; Vol. 42, issue Suppl 57:145s [P761]. [CENTRAL: 973521; CRS: 4900126000006741; EMBASE: 71842512]
-
- Maltais F, Singh S, Donald A, Crater G, Church A, Goh A. Erratum: effects of a combination of umeclidinium/vilanterol on exercise endurance in patients with chronic obstructive pulmonary disease: two randomised, double‐blind clinical trials (Therapeutic Advances in Respiratory Disease (2014) 8 (169‐181)). Therapeutic Advances in Respiratory Disease 2016;10(3):289. [CRS: 4900132000025878; EMBASE: 20160439624] - PubMed
-
- Maltais F, Singh S, Donald AC, Crater G, Church A, Goh AH, et al. Effects of a combination of umeclidinium/vilanterol on exercise endurance in patients with chronic obstructive pulmonary disease: two randomised, double‐blind clinical trials. Therapeutic Advances in Respiratory Disease 2014;8(6):169‐81. [CENTRAL: 1020063; CRS: 4900126000022533; PUBMED: 25452426] - PubMed
-
- NCT01328444. An exercise endurance study to evaluate the effects of treatment of chronic obstructive pulmonary disease (COPD) patients with a dual bronchodilator: GSK573719/GW642444. Study A. clinicaltrials.gov/show/NCT01328444 (first received 1 April 2011). [CRS: 4900132000005779]
Maltais 2014a {published and unpublished data}
-
- DB2114418. An exercise endurance study to evaluate the effects of treatment of chronic obstructive pulmonary disease (COPD) patients with a dual bronchodilator: GSK573719/GW642444. Study B. http://www.gsk‐clinicalstudyregister.com/files/114418/2627/gsk‐114418‐clinical‐study‐re... (first received 16 March 2011).
-
- Maltais F, Singh S, Donald A, Church A, Crater G, Goh A, et al. Effects of a combination of vilanterol and umeclidinium on exercise endurance in subjects with COPD: two randomised clinical trials [Abstract]. American Thoracic Society International Conference; 2013 May 17‐22; Philadelphia. 2013; Vol. 42, issue Suppl 57:145s [P761]. [CENTRAL: 973521; CRS: 4900126000006741; EMBASE: 71842512]
-
- Maltais F, Singh S, Donald A, Crater G, Church A, Goh A. Erratum: effects of a combination of umeclidinium/vilanterol on exercise endurance in patients with chronic obstructive pulmonary disease: two randomised, double‐blind clinical trials (Therapeutic Advances in Respiratory Disease (2014) 8 (169‐181)). Therapeutic Advances in Respiratory Disease 2016;10(3):289. [CRS: 4900132000025878; EMBASE: 20160439624] - PubMed
-
- Maltais F, Singh S, Donald AC, Crater G, Church A, Goh AH, et al. Effects of a combination of umeclidinium/vilanterol on exercise endurance in patients with chronic obstructive pulmonary disease: two randomised, double‐blind clinical trials. Therapeutic Advances in Respiratory Disease 2014;8(6):169‐81. [CENTRAL: 1020063; CRS: 4900126000022533; PUBMED: 25452426] - PubMed
-
- NCT01323660. An exercise endurance study to evaluate the effects of treatment of chronic obstructive pulmonary disease (COPD) patients with a dual bronchodilator: GSK573719/GW642444. Study B. clinicaltrials.gov/show/NCT01323660 (first received 24 March 2011). [CRS: 4900132000005798]
Mehta 2011 {published and unpublished data}
-
- AC4108123. A randomised, double blind, placebo‐controlled, double dummy, 4‐way cross‐over, dose ascending study to assess the safety, tolerability, pharmacodynamics and pharmacokinetics of single inhaled doses of GSK573719 (250, 500 and 1000 μg) and tiotropium bromide (18 μg) via DPI in COPD patients. http://www.gsk‐clinicalstudyregister.com/files/AC4108123/4874/gsk‐ac4108123‐clinical‐st... (first received 13 September 2012).
-
- Mehta R, Newlands A, Kelleher D, Preece A, Cahn A, Crater G. Safety, pharmacokinetics (PK) and pharmacodynamics (PD) of single doses of GSK573719 inhalation powder, a new long‐acting muscarinic antagonist (LAMA), in patients with COPD [Abstract]. European Respiratory Society Annual Congress, Amsterdam, the Netherlands, September 24‐28. 2011; Vol. 38, issue 55:138s [P822]. [CENTRAL: 833461; CRS: 4900100000053965]
-
- NCT00515502. Safety study using GSK573719 and tiotropium in patients with chronic obstructive pulmonary disease. clinicaltrials.gov/show/NCT00515502 (first received 9 August 2007). [CRS: 4900132000005764]
-
- Tal‐Singer R, Cahn A, Mehta R, Preece A, Crater G, Kelleher D, et al. Initial assessment of single and repeat doses of inhaled umeclidinium in patients with chronic obstructive pulmonary disease: two randomised studies. European Journal of Pharmacology 2013;701(1‐3):40‐8. [CENTRAL: 853353; CRS: 4900100000074852; EMBASE: 2013146506; PUBMED: 23276660] - PubMed
Mehta 2013 {published and unpublished data}
-
- DB2113950. A single‐centre, randomised, open‐label study to evaluate the effects of steady‐state verapamil, a moderate P‐glycoprotein and CYP3A4 inhibitor, on the pharmacokinetics of GSK573719 and GSK573719 in combination with GW642444. http://www.gsk‐clinicalstudyregister.com/files/113950/5554/gsk‐113950‐clinical‐study‐re... (first received 13 September 2010).
-
- Mehta R, Kelleher D, Hughes S, Preece A, Crater G. Evaluation of the effect of verapamil, an inhibitor of P‐glycoprotein and CYP3A4, on systemic exposure and safety profile of GSK573719, a new long‐acting muscarinic antagonist and vilanterol, an inhaled long‐acting beta2 agonist, in healthy adults [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185(Meeting Abstracts):A2917. [CENTRAL: 834322; CRS: 4900100000060590]
-
- Mehta R, Kelleher D, Preece A, Hughes S, Crater G. Effect of verapamil on systemic exposure and safety of umeclidinium and vilanterol: a randomised and open‐label study. International Journal of Chronic Obstructive Pulmonary Disease 2013;8:159‐67. [CENTRAL: 849002; CRS: 4900100000073942; EMBASE: 2013209218; PUBMED: 23569370] - PMC - PubMed
-
- NCT01128634. Pharmacokinetic and safety of GSK573719 and GW642444 administered individually and concurrently, with verapamil in healthy subjects. clinicaltrials.gov/ct2/show/NCT01128634 (first received 20 May 2010).
Mehta 2013a {published and unpublished data}
-
- AC4115487. Randomised, double‐blind, 5 period cross‐over study assessing lung function in healthy volunteers following single inhalations of GSK573719 inhalation powder from two configurations of the novel dry powder inhaler. http://www.gsk‐clinicalstudyregister.com/files2/fff6ad8a‐fa02‐444a‐aeee‐e179b5298fde (first received 12 October 2011).
-
- Mehta R, Arzoz L, Fayinka S, Preece A, Crater G, Tombs L, et al. Pharmacodynamic and pharmacokinetic performance of two configurations of a new dry powder inhaler developed for administration of umeclidinium. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A4360. [CENTRAL: 870747; CRS: 4900100000087892]
-
- NCT01521390. Assessment of lung function after single inhalations of a bronchodilator from 2 configurations of a dry powder inhaler. clinicaltrials.gov/ct2/show/NCT01521390 (first received 26 January 2012).
Mehta 2014 {published and unpublished data}
-
- DB2114637. An open‐label, non‐randomised, pharmacokinetic and safety study of single dose GSK573719 + GW643444 (VI) combination and repeat doses of GSK573719 in healthy subjects and in subjects with moderate hepatic impairment. gsk‐clinicalstudyregister.com/files/114637/3973/gsk‐114637‐clinical‐study‐re... (first received 29 October 2012).
-
- Mehta R, Hardes K, Kelleher D, Preece A, Tombs L, Brealey N. Effects of moderate hepatic impairment on the pharmacokinetic properties and tolerability of umeclidinium and vilanterol in inhalational umeclidinium monotherapy and umeclidinium/vilanterol combination therapy: an open‐label, non‐randomised study. Clinical Therapeutics 2014;36(7):1016‐27.e2. [PUBMED: 24947493] - PubMed
-
- NCT01577680. A study to assess the effects of GSK573719/VI combination and GSK573719 monotherapy in subjects with moderate hepatic impairment and matched healthy volunteers [An open‐label, non‐randomised, pharmacokinetic and safety study of single dose GSK573719 + GW643444 (VI) combination and repeat doses of GSK573719 in healthy subjects and in subjects with moderate hepatic impairment]. clinicaltrials.gov/ct2/show/NCT01577680 (first received 29 March 2012).
Minakata 2014 {published and unpublished data}
-
- DB2115362. A 52‐week, multi‐centre, open‐label study to evaluate the safety and tolerability of GSK573719 125 mcg once‐daily in combination with GW642444 25 mcg once‐daily via novel dry powder inhaler (nDPI) in Japanese subjects with chronic obstructive pulmonary disease. http://www.gsk‐clinicalstudyregister.com/files2/8e798279‐a8b0‐4fa3‐84f6‐ff747c5b8dc2 (first received 2 August 2011).
-
- Minakata Y, Saotome T, Mihara K, Hashimoto K. Long‐term treatment study of umeclidinium/vilanterol combination (UMEC/VI) in Japanese patients with COPD. Respiratory Medicine 2014;10:1037‐47.
-
- NCT01376388. Long‐term safety study for GSK573719/GW642444 in Japanese (DB2115362) [A 52‐week, multi‐centre, open‐label study to evaluate the safety and tolerability of GSK573719 125 mcg once‐daily in combination with GW642444 25 mcg once‐daily via novel dry powder inhaler (nDPI) in Japanese subjects with chronic obstructive pulmonary disease]. clinicaltrials.gov/show/NCT01376388 (first received 9 June 2011). [CRS: 4900132000005789]
Nakahara 2012 {published and unpublished data}
-
- AC4113377. Phase I study of GSK573719 ‐ a randomised, double blind, placebo controlled, dose ascending, single and repeat dose study to investigate the safety, tolerability, and pharmacokinetics of inhaled dose of GSK573719 from a novel dry powder device in healthy Japanese male subjects. http://www.gsk‐clinicalstudyregister.com/files/113377/5042/gsk‐113377‐clinical‐study‐re... (first received 25 November 2011).
-
- NCT01013974. A study of GSK573719 in healthy Japanese male subjects [Phase I study of GSK573719 ‐ a randomised, double blind, placebo controlled, dose ascending, single and repeat dose study to investigate the safety, tolerability, and pharmacokinetics of inhaled dose of GSK573719 from a novel dry powder device in healthy Japanese male subjects]. clinicaltrials.gov/ct2/show/NCT01013974 (first received 12 November 2009).
-
- Nakahara N, Takahashi N, Kelleher D, Mehta R. Safety, tolerability and pharmacokinetics (PK) of single and repeat doses of GSK573719 in healthy Japanese subjects [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185(Meeting Abstracts):A2915. [CENTRAL: 834280; CRS: 4900100000060548]
NCT01110018 {unpublished data only}
-
- AC4112008. A single‐centre, open‐label, sequential, cross‐over study to examine the safety, tolerability and pharmacokinetics of 3 ascending single intravenous doses, a single 1000μg oral dose and a single 1000μg inhaled dose of GSK573719 in healthy male volunteers. gsk‐clinicalstudyregister.com/files/112008/3795/gsk‐112008‐clinical‐study‐re... (first received 22 February 2011).
-
- NCT01110018. GSK573719 IV enabling study [A single‐centre, open‐label, sequential, cross‐over study to examine the safety, tolerability and pharmacokinetics of 3 ascending single intravenous doses, a single 1000μg oral dose and a single 1000μg inhaled dose of GSK573719 in healthy male volunteers]. clinicaltrials.gov/show/NCT01110018 (first received 15 April 2010). [CRS: 4900132000005767]
NCT01491802 {unpublished data only}
-
- NCT01491802. Effect of a new combination bronchodilator on exercise in GOLD Stage II moderate COPD [A 4‐week randomised, double‐blind, cross‐over study to assess the effect of a new LABA/LAMA combination versus LAMA alone on exertional dyspnea, exercise endurance and neuromechanical coupling in patients with GOLD stage II COPD]. clinicaltrials.gov/show/NCT01491802 (first received 12 December 2011). [CRS: 4900132000005796]
NCT01571999 {unpublished data only}
-
- NCT01571999. Study to assess the safety and PK of GSK573719 and GSK573719/GW642444(VI) combination in healthy subjects and subjects with severe renal impairment [A single‐blind, non‐randomised pharmacokinetic and safety study of single dose of GSK573719 and GSK573719 + GW642444 combination in healthy subjects and in subjects with severe renal impairment]. clinicaltrials.gov/show/NCT01571999 (first received 3 April 2012). [CRS: 4900132000005783]
NCT01636713 {unpublished data only}
-
- NCT01636713. A 24‐week study to evaluate the efficacy and safety of GSK573719/GW642444 125/25 mcg and 62.5/25mcg inhalation powder compared with placebo in subjects with COPD [A 24‐week randomised, double‐blind and placebo‐controlled study to evaluate the efficacy and safety of GSK573719/GW642444 125/25 mcg and 62.5/25mcg inhalation powder compared with placebo inhalation powder delivered once‐daily via a novel dry powder inhaler in subjects with chronic obstructive pulmonary disease (COPD)]. clinicaltrials.gov/show/NCT01636713 (first received 5 July 2012). [CRS: 4900132000005773]
NCT01725685 {unpublished data only}
-
- NCT01725685. To investigate the pharmacokinetics and safety of fluticasone furoate (FF)/ umeclidinium (UMEC) combination compared with FF and UMEC monotherapies in adult healthy volunteers using a dry powder inhaler (DPI) [A randomised, double‐blind, single‐dose, three‐period, crossover study to investigate pharmacokinetic, safety and tolerability of fluticasone furoate with umeclidinium when administered in combination and as monotherapies in adult healthy volunteer subjects]. clinicaltrials.gov/ct2/show/NCT01725685 (first received 1 November 2012).
NCT02257385 {unpublished data only}
-
- NCT02257385. Comparative study of umeclidinium/vilanterol (UMEC/VI) in a fixed dose combination with indacaterol plus tiotropium [Study DB2116961, A multicentre, randomised, blinded, parallel group study to compare UMEC/VI (umeclidinium/vilanterol) in a fixed dose combination with indacaterol plus tiotropium in symptomatic subjects with moderate to very severe COPD]. clinicaltrials.gov/show/NCT02257385 (first received 2 October 2014). [CRS: 4900132000005805]
NCT02275052 {unpublished data only}
-
- 201317. A randomised, double‐blind, placebo‐controlled evaluation of the effect of the combination of umeclidinium and vilanterol on exercise endurance time in subjects with COPD. http://www.gsk‐clinicalstudyregister.com/study/201317?search=compound&compound=umec... (first received January 2015).
-
- NCT02275052. A study to evaluate the effect of the combination of umeclidinium (UMEC) and vilanterol (VI) on exercise endurance time (EET) in participants with chronic obstructive pulmonary disease (COPD) [A randomised, double‐blind, placebo‐controlled evaluation of the effect of the combination of umeclidinium and vilanterol on exercise endurance time in subjects with COPD]. clinicaltrials.gov/show/NCT02275052 (first received 23 October 2014). [CRS: 4900132000005760]
NCT02487446 {unpublished data only}
-
- NCT02487446. Efficacy and safety study of QVA149 in COPD patients. 2015, https://clinicaltrials.gov/show/NCT02487446. [CRS: 4900132000042544]
NCT02487498 {unpublished data only}
-
- NCT02487498. Efficacy and safety study of indacaterol maleate/glycopyrronium bromide in chronic obstructive pulmonary disease (COPD) patients. [A multi‐centre, randomised, double‐blind, double‐dummy, active controlled, two‐period cross‐over study to assess the efficacy, safety and tolerability of indacaterol maleate/glycopyrronium bromide compared to umeclidinium bromide/vilanterol in COPD patients with moderate to severe airflow limitation]. clinicaltrials.gov/show/NCT02487498 (first received 29 June 2015). [CRS: 4900132000005797]
NCT02570165 {unpublished data only}
-
- 201012. A dose‐finding study of batefenterol (GSK961081) via dry powder inhaler in patients with COPD. gsk‐clinicalstudyregister.com/study/201012#ps (first received November 2015).
-
- EUCTR2015‐001409‐15‐DE. Study 201012: a dose‐finding study of batefenterol (GSK961081) via dry powder inhaler in patients with COPD. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015‐001409‐15‐DE (first received 21 August 2015).
-
- NCT02570165. Study 201012: a dose‐finding study of batefenterol (GSK961081) via dry powder inhaler in patients with COPD. clinicaltrials.gov/show/NCT02570165 (first received 5 October 2015). [CRS: 4900132000032161]
NCT02729051 {unpublished data only}
-
- 200812. A phase IIIB, 24‐week randomised, double‐blind study to compare ‘closed’ triple therapy (FF/UMEC/VI) with 'open' triple therapy (FF/VI + UMEC), in subjects with chronic obstructive pulmonary disease (COPD). https://www.gsk‐clinicalstudyregister.com/study/200812#ps.
-
- EUCTR2015‐005212‐14‐ES. A 24 week study comparing 'closed' triple therapy delivered as FF/UMEC/VI vs 'open' triple therapy delivered as FF/VI + UMEC in COPD patients. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015‐005212‐14‐ES (first received 24 February 2016).
-
- EUCTR2015‐005212‐14‐FR. A phase IIIB, 24‐week randomised, double‐blind study to compare ‘closed’ triple therapy (FF/UMEC/VI) with 'open' triple therapy (FF/VI + UMEC), in subjects with chronic obstructive pulmonary disease (COPD). apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015‐005212‐14‐FR (first received 20 April 2016).
-
- NCT02729051. Comparative study of fluticasone furoate(FF)/umeclidinium bromide (UMEC)/vilanterol (VI) closed therapy versus FF/VI Plus UMEC open therapy in subjects with chronic obstructive pulmonary disease (COPD) [A phase IIIB, 24‐week randomised, double‐blind study to compare ‘closed’ triple therapy (FF/UMEC/VI) with 'open' triple therapy (FF/VI + UMEC), in subjects with chronic obstructive pulmonary disease (COPD)]. clinicaltrials.gov/show/NCT02729051 (first received 31 March 2016).
NCT02731846 {unpublished data only}
-
- NCT02731846. A study comparing the closed triple therapy, open triple therapy and a dual therapy for effect on lung function in subjects with chronic obstructive pulmonary disease (COPD) [A phase III, 4‐week, randomised, double‐blind study to compare 'closed' triple therapy (FF/UMEC/VI), 'open' triple therapy (FF/VI + UMEC) and dual therapy (FF/VI) in subjects with chronic obstructive pulmonary disease (COPD)]. clinicaltrials.gov/show/NCT02731846 (first received 4 April 2016). [CRS: 4900132000032162]
NCT02799784 {unpublished data only}
-
- 204990. A randomised, open‐label, 8‐week cross‐over study to compare umeclidinium/vilanterol with tiotropium/olodaterol once‐daily in subjects with chronic obstructive pulmonary disease (COPD). gsk‐clinicalstudyregister.com/study/204990#ps (first received July 2016).
-
- EUCTR2016‐000585‐36‐DE. A randomised, open‐label, 8‐week cross‐over study to compare umeclidinium/vilanterol with tiotropium/olodaterol once‐daily in subjects with chronic obstructive pulmonary disease (COPD). apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2016‐000585‐36‐DE (first received 17 May 2016).
-
- NCT02799784. An efficacy study of umeclidinium/vilanterol with tiotropium/olodaterol in COPD patients [A randomised, open‐label, 8‐week cross‐over study to compare umeclidinium/vilanterol with tiotropium/olodaterol once‐daily in subjects with chronic obstructive pulmonary disease (COPD)]. clinicaltrials.gov/ct2/show/NCT02799784 (first received 2 June 2016).
NCT02837380 {unpublished data only}
-
- 200558. An open label study to evaluate the pharmacokinetics of fluticasone furoate/umeclidinium bromide/vilanterol (100/62.5/25 mcg) after single and repeat dose administration from a dry powder inhaler in healthy Chinese subjects. https://www.gsk‐clinicalstudyregister.com/study/200558#ps.
-
- NCT02837380. A phase I pharmacokinetic study of fluticasone furoate/umeclidinium bromide/vilanterol (100/62.5/25 microgram [mcg]) after single and repeat dose administration from a dry powder inhaler in healthy Chinese subjects. clinicaltrials.gov/ct2/show/NCT02837380 (first received 15 July 2016).
NCT03034915 {unpublished data only}
-
- 201749. A 24‐week treatment, multi‐centre, randomised, double‐blind, double‐dummy, parallel group study to compare umeclidinium/vilanterol, umeclidinium, and salmeterol in subjects with chronic obstructive pulmonary disease (COPD). https://www.gsk‐clinicalstudyregister.com/study/201749#ps.
-
- EUCTR2016‐002513‐22‐ES. A 24‐week treatment, multi‐centre, randomised, double‐blind, double‐dummy, parallel group study to compare umeclidinium/vilanterol, umeclidinium, and salmeterol in subjects with chronic obstructive pulmonary disease (COPD). http://apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2016‐002513‐22‐ES.
-
- NCT03034915. A 24‐week study to compare umeclidinium/vilanterol (UMEC/VI), UMEC and salmeterol in subjects with chronic obstructive pulmonary disease (COPD). https://clinicaltrials.gov/show/NCT03034915 2017. [CRS: 4900132000042577]
Pascoe 2016 {published and unpublished data}
-
- CTT116855. A phase III, 52 week, randomised, double‐blind, 3‐arm parallel group study, comparing the efficacy, safety and tolerability of the fixed dose triple combination FF/UMEC/VI with the fixed dose dual combinations of FF/VI and UMEC/VI, all administered once‐daily in the morning via a dry powder inhaler in subjects with chronic obstructive pulmonary disease. gsk‐clinicalstudyregister.com/study/116855#ps (first received June 2014).
-
- EUCTR2013‐003075‐35‐NL. A phase III, 52 week, randomised, double‐blind, 3‐arm parallel group study, comparing the efficacy, safety and tolerability of the fixed dose triple combination fluticasone furoate/umeclidinium/vilanterol, with the fixed dose dual combinations of fluticasone furoate/vilanterol and umeclidinium/vilanterol, all administered once‐daily in the morning via a dry powder inhaler in subjects with chronic obstructive pulmonary disease. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013‐003075‐35‐NL (first received 24 July 2014).
-
- NCT02164513. A study comparing the efficacy, safety and tolerability of fixed dose combination (FDC) of FF/UMEC/VI with the FDC of FF/VI and UMEC/VI; administered once‐daily via a dry powder inhaler (DPI) in subjects with chronic obstructive pulmonary disease (COPD) [A phase III, 52 week, randomised, double‐blind, 3‐arm parallel group study, comparing the efficacy, safety and tolerability of the fixed dose triple combination FF/UMEC/VI with the fixed dose dual combinations of FF/VI and UMEC/VI, all administered once‐daily in the morning via a dry powder inhaler in subjects with chronic obstructive pulmonary disease]. clinicaltrials.gov/show/NCT02164513 (first received 12 June 2014). [CRS: 4900132000005795]
-
- PER‐042‐14. A phase III, 52 week, randomised, double‐blind, 3‐arm parallel group study, comparing the efficacy, safety and tolerability of the fixed dose triple combination FF/UMEC/VI with the fixed dose dual combinations of FF/VI and UMEC/VI, all administered once‐daily in the morning via a dry powder inhaler in subjects with chronic obstructive pulmonary disease. apps.who.int/trialsearch/Trial2.aspx?TrialID=PER‐042‐14 (first received 4 December 2014).
-
- Pascoe SJ, Lipson DA, Locantore N, Barnacle H, Brealey N, Mohindra R, et al. A phase III randomised controlled trial of single‐dose triple therapy in COPD: the IMPACT protocol. European Respiratory Journal 2016;48(2):320‐30. [CRS: 4900132000026576; EMBASE: 20160571871; PUBMED: 27418551] - PubMed
Rheault 2016 {published and unpublished data}
-
- 201315. A randomised, parallel‐group, open‐label study to evaluate the efficacy and safety of umeclidinium (UMEC) 62.5 mcg compared with glycopyrronium 44 mcg in subjects with chronic obstructive pulmonary disease (COPD). gsk‐clinicalstudyregister.com/files2/gsk‐201315‐Clinical‐Study‐Result‐Summar... (first received 26 September 2014).
-
- EUCTR2014‐000885‐23‐SE. A randomised, parallel‐group, open‐label study to evaluate the efficacy and safety of umeclidinium (UMEC) 62.5 mcg compared with glycopyrronium 44 mcg in subjects with chronic obstructive pulmonary disease (COPD). apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2014‐000885‐23‐SE (first received 14 May 2014).
-
- NCT02236611. A 12‐week study to evaluate the efficacy and safety of umeclidinium 62.5 microgram (mcg) compared with glycopyrronium 44 mcg in subjects with chronic obstructive pulmonary disease (COPD) [A randomised, parallel‐group, open‐label study to evaluate the efficacy and safety of umeclidinium (UMEC) 62.5 mcg compared with glycopyrronium 44 mcg in subjects with chronic obstructive pulmonary disease (COPD)]. clinicaltrials.gov/show/NCT02236611 (first received 8 September 2014). [CRS: 4900132000005784]
Siler 2015 {published and unpublished data}
-
- 200109. A study to compare the addition of umeclidinium bromide (UMEC) to fluticasone furoate (FF)/vilanterol (VI), with placebo plus FF/VI in subjects with chronic obstructive pulmonary disease (COPD) ‐ Study 1. gsk‐clinicalstudyregister.com/files/200109/6024/gsk‐200109‐clinical‐study‐re... (first received 4 October 2013).
-
- EUCTR2013‐002238‐19‐RO. Umeclidinium bromide added onto fluticasone furoate/vilanterol in COPD – Study 1 [A study to compare the addition of umeclidinium bromide (UMEC) to fluticasone furoate (FF)/vilanterol (VI), with placebo plus FF/VI in subjects with chronic obstructive pulmonary disease (COPD) ‐ Study 1]. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013‐002238‐19‐RO (first received 5 November 2013).
-
- NCT01957163. A study to compare the addition of umeclidinium bromide (UMEC) to fluticasone furoate (FF)/vilanterol (VI), with placebo plus FF/VI in subjects with chronic obstructive pulmonary disease (COPD) ‐ Study 1. clinicaltrials.gov/show/NCT01957163 (first received 4 October 2013). [CRS: 4900132000005780]
-
- Siler T, Kerwin E, Sousa A, Donald A, Ali R, Church A. Efficacy and safety of once‐daily umeclidinium added to fluticasone furoate/vilanterol in chronic obstructive pulmonary disease: results of two replicate randomised 12‐week studies. Chest 2014;146(4):MEETING ABSTRACT. [CENTRAL: 1051009; CRS: 4900126000026305; EMBASE: 71780470]
-
- Siler TM, Kerwin E, Sousa AR, Donald A, Ali R, Church A. Efficacy and safety of umeclidinium added to fluticasone furoate/vilanterol in chronic obstructive pulmonary disease: results of two randomised studies. Respiratory Medicine 2015;109(9):1155‐63. [CENTRAL: 1077140; CRS: 4900132000004607; PUBMED: 26117292] - PubMed
Siler 2015a {published and unpublished data}
-
- 200110. A study to compare the addition of umeclidinium bromide (UMEC) to fluticasone furoate (FF)/vilanterol (VI), with placebo plus FF/VI in subjects with chronic obstructive pulmonary disease (COPD) ‐ Study 2. gsk‐clinicalstudyregister.com/files/200110/6027/gsk‐200110‐clinical‐study‐re... (first received 16 October 2013).
-
- EUCTR2013‐002239‐44‐DE. A study to compare the addition of umeclidinium bromide (UMEC) to fluticasone furoate (FF)/vilanterol (VI), with placebo plus FF/VI in subjects with chronic obstructive pulmonary disease (COPD) ‐ Study 2. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013‐002239‐44‐DE (first received 15 July 2013).
-
- NCT02119286. A study to compare the addition of umeclidinium bromide (UMEC) to fluticasone furoate (FF)/vilanterol (VI), with placebo plus FF/VI in subjects with chronic obstructive pulmonary disease (COPD) ‐ Study 2. clinicaltrials.gov/show/NCT02119286 (first received 17 April 2014). [CRS: 4900132000005768]
-
- Siler T, Kerwin E, Sousa A, Donald A, Ali R, Church A. Efficacy and safety of once‐daily umeclidinium added to fluticasone furoate/vilanterol in chronic obstructive pulmonary disease: results of two replicate randomised 12‐week studies. Chest 2014;146(4):MEETING ABSTRACT. [CENTRAL: 1051009; CRS: 4900126000026305; EMBASE: 71780470]
-
- Siler TM, Kerwin E, Sousa AR, Donald A, Ali R, Church A. Efficacy and safety of umeclidinium added to fluticasone furoate/vilanterol in chronic obstructive pulmonary disease: results of two randomised studies. Respiratory Medicine 2015;109(9):1155‐63. [CENTRAL: 1077140; CRS: 4900132000004607; PUBMED: 26117292] - PubMed
Siler 2016 {published and unpublished data}
-
- AC4116136. A multicentre, randomised, double‐blind, parallel group study to evaluate the efficacy and safety of the addition of umeclidinium bromide inhalation powder (62.5mcg) once‐daily to fluticasone propionate/salmeterol (250/50mcg) twice‐daily, umeclidinium bromide inhalation powder (125mcg) once‐daily to fluticasone propionate/salmeterol (250/50mcg) twice‐daily versus placebo to fluticasone propionate/salmeterol (250/50mcg) twice‐daily over 12 weeks in subjects with COPD. gsk‐clinicalstudyregister.com/files2/116136‐Clinical‐Study‐Result‐Summary.pdf (first received 23 January 2013).
-
- EUCTR2012‐001871‐35‐CZ. AC4116136: a multicentre, randomised, double‐blind, parallel group study to evaluate the efficacy and safety of the addition of umeclidinium bromide inhalation powder (62.5mcg) once‐daily to fluticasone propionate/salmeterol (250/50mcg) twice‐daily, umeclidinium bromide inhalation powder (125mcg) once‐daily to fluticasone propionate/salmeterol (250/50mcg) twice‐daily versus placebo to fluticasone propionate/salmeterol (250/50mcg) twice‐daily over 12 weeks in subjects with COPD. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2012‐001871‐35‐CZ (first received 20 December 2012).
-
- Kerwin E, Shah P, Singletary K, Church A. Efficacy and safety of umeclidinium added to fluticasone propionate/salmeterol in patients with COPD: results of a randomised, double‐blind study (Abstract). American Journal of Respiratory and Critical Care Medicine 2014;189:A3769. [CENTRAL: 1035578; CRS: 4900126000023087]
-
- NCT01772147. Efficacy and safety of the addition of fluticasone propionate/salmeterol (250/50mcg) twice‐daily to 2 doses of umeclidinium bromide inhalation powder (62.5 or 125mcg) once‐daily over 12 weeks. [A multicentre, randomised, double‐blind, parallel group study to evaluate the efficacy and safety of the addition of umeclidinium bromide inhalation powder (62.5mcg) once‐daily to fluticasone propionate/salmeterol (250/50mcg) twice‐daily, umeclidinium bromide inhalation powder (125mcg) once‐daily to fluticasone propionate/salmeterol (250/50mcg) twice‐daily versus placebo to fluticasone propionate/salmeterol (250/50mcg) twice‐daily over 12 weeks in subjects with COPD]. clinicaltrials.gov/show/NCT01772147 (first received 17 January 2013). [CRS: 4900132000005803]
-
- Siler TM, Kerwin E, Singletary K, Brooks J, Church A. Efficacy and safety of umeclidinium added to fluticasone propionate/salmeterol in patients with COPD: results of two randomised, double‐blind studies. COPD 2016;13(1):1‐10. [CENTRAL: 1133509; CRS: 4900132000016001; EMBASE: 2015458038] - PMC - PubMed
Siler 2016a {published and unpublished data}
-
- AC4116135. A multicentre, randomised, double‐blind, parallel‐group study to evaluate the efficacy and safety of the addition of umeclidinium bromide (62.5mcg) once‐daily to fluticasone propionate/salmeterol (250/50mcg) twice‐daily, umeclidinium bromide (125mcg) once‐daily to fluticasone propionate/salmeterol (250/50mcg) twice‐daily versus placebo to fluticasone propionate/salmeterol (250/50mcg) twice‐daily over 12 weeks with COPD. gsk‐clinicalstudyregister.com/files2/116135‐Clinical‐Study‐Result‐Summary.pdf (first received 24 January 2013).
-
- NCT01772134. Efficacy and safety of the addition of fluticasone propionate/salmeterol (250/50mcg) twice‐daily to 2 doses of umeclidinium bromide (62.5 or 125mcg) once‐daily over 12 weeks [A multicentre, randomised, double‐blind, parallel‐group study to evaluate the efficacy and safety of the addition of umeclidinium bromide (62.5mcg) once‐daily to fluticasone propionate/salmeterol (250/50mcg) twice‐daily, umeclidinium bromide (125mcg) once‐daily to fluticasone propionate/salmeterol (250/50mcg) twice‐daily versus placebo to fluticasone propionate/salmeterol (250/50mcg) twice‐daily over 12 weeks with COPD]. clinicaltrials.gov/show/NCT01772134 (first received 17 January 2013). [CRS: 4900132000005782]
-
- Siler TM, Kerwin E, Singletary K, Brooks J, Church A. Efficacy and safety of umeclidinium added to fluticasone propionate/salmeterol in patients with COPD: results of two randomised, double‐blind studies. COPD 2016;13(1):1‐10. [CENTRAL: 1133509; CRS: 4900132000016001; EMBASE: 2015458038] - PMC - PubMed
Siler 2016b {published and unpublished data}
-
- 201211. A 12 week, multicentre, randomised, double‐blind, parallel‐group, placebo‐controlled study to evaluate the efficacy of umeclidinium/vilanterol 62.5/25mcg in subjects with COPD. gsk‐clinicalstudyregister.com/files2/201211‐Clinical‐Study‐Result‐Summary.pdf (first received 15 September 2014).
-
- EUCTR2014‐000529‐19‐HU. A 12 week, multicentre, randomised, double‐blind, parallel‐group, placebo‐controlled study to evaluate the efficacy of umeclidinium/vilanterol 62.5/25mcg in subjects with COPD. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2014‐000529‐19‐HU (first received 11 April 2014).
-
- NCT02152605. A phase IIIb study to evaluate the efficacy of umeclidinium/vilanterol (UMEC/VI) in subjects with chronic obstructive pulmonary disease (COPD) [A 12 week, multicentre, randomised, double‐blind, parallel‐group, placebo‐controlled study to evaluate the efficacy of umeclidinium/vilanterol 62.5/25mcg in subjects with COPD]. clinicaltrials.gov/show/NCT02152605 (first received 29 May 2014). [CRS: 4900132000005762]
-
- Siler TM, Donald AC, O'Dell D, Church A, Fahy WA. A randomised, parallel‐group study to evaluate the efficacy of umeclidinium/vilanterol 62.5/25 mug on health‐related quality of life in patients with COPD. International Journal of Chronic Obstructive Pulmonary Disease 2016;11:971‐9. [PUBMED: 27274218] - PMC - PubMed
Singh 2015 {published and unpublished data}
-
- DB2116134. A randomised, multi‐centre, double‐blind, double dummy, parallel group study to evaluate the efficacy and safety of umeclidinium bromide/vilanterol compared with fluticasone propionate/salmeterol over 12 weeks in subjects with COPD. gsk‐clinicalstudyregister.com/files2/116134‐Clinical‐Study‐Result‐Summary.pdf (first received 3 April 2013).
-
- EUCTR2012‐000524‐18‐CZ. A randomised, multi‐centre, double‐blind, double dummy, parallel group study to evaluate the efficacy and safety of umeclidinium bromide/vilanterol compared with fluticasone propionate/salmeterol over 12 weeks in subjects with COPD. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2012‐000524‐18‐CZ (first received 20 December 2012).
-
- NCT01822899. A study to evaluate the efficacy and safety of umeclidinium bromide/vilanterol compared with fluticasone propionate/salmeterol over 12 weeks in subjects with chronic obstructive pulmonary disease (COPD) [DB2116134: a randomised, multi‐centre, double‐blind, double dummy, parallel group study to evaluate the efficacy and safety of umeclidinium bromide/vilanterol compared with fluticasone propionate/salmeterol over 12 weeks in subjects with COPD]. clinicaltrials.gov/show/NCT01822899 (first received 28 March 2013). [CRS: 4900132000005787]
-
- Singh D, Worsley S, Zhu C‐Q, Hardaker L, Church A. Umeclidinium/vilanterol (UMEC/VI) once daily (OD) vs fluticasone/salmeterol combination (FSC) twice daily (BD) in patients with moderate‐to‐severe COPD and infrequent COPD exacerbations [Abstract]. European Respiratory Journal 2014;44(Suppl 58):P290. [CENTRAL: 1053468; CRS: 4900126000028668; EMBASE: 71849975]
Sousa 2016 {published and unpublished data}
-
- 201314. A randomised, parallel group study to evaluate the effect of umeclidinium (UMEC) added to inhaled corticosteroid/long‐acting beta‐agonist combination therapy in subjects with chronic obstructive pulmonary disease COPD. gsk‐clinicalstudyregister.com/files2/201314‐Clinical‐Study‐Result‐Summary.pdf (first received 30 September 2014).
-
- EUCTR2014‐000611‐14‐NL. A randomised, parallel group study to evaluate the effect of umeclidinium (UMEC) added to inhaled corticosteroid/ long‐acting beta‐agonist combination therapy in subjects with chronic obstructive pulmonary disease COPD. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2014‐000611‐14‐NL (first received 27 June 2014).
-
- NCT02257372. A study to evaluate the effect of umeclidinium (UMEC) as combination therapy in subjects with chronic obstructive pulmonary disease (COPD) [A randomised, parallel group study to evaluate the effect of umeclidinium (UMEC) added to inhaled corticosteroid/ long‐acting beta‐agonist combination therapy in subjects with chronic obstructive pulmonary disease COPD]. clinicaltrials.gov/show/NCT02257372 (first received 2 October 2014). [CRS: 4900132000005776]
-
- Sousa AR, Riley JH, Church A, Zhu CQ, Punekar YS, Fahy WA. A randomised, parallel‐group study to evaluate the effect of umeclidinium added to inhaled corticosteroid/long‐acting beta‐agonist combination therapy in subjects with chronic obstructive pulmonary disease. Thorax 2015;70:A137‐8. [CENTRAL: 1135261; CRS: 4900132000016622; EMBASE: 72199706]
-
- Sousa AR, Riley JH, Church A, Zhu CQ, Punekar YS, Fahy WA. The effect of umeclidinium added to inhaled corticosteroid/long‐acting beta2‐agonist in patients with symptomatic COPD: a randomised, double‐blind, parallel‐group study. NPJ Primary Care Respiratory Medicine 2016;26:16031. [CENTRAL: 1161147; CRS: 4900132000025438; EMBASE: 20160474127; PUBMED: 27334739] - PMC - PubMed
Webb 2015 {published data only}
-
- Webb KA, Ciavaglia CE, Preston M, O'Donnell DE. Effects of dual umeclidinium/vilanterol compared to umeclidinium on exertional dyspnea, respiratory mechanics and neural drive in patients with moderate COPD. American Journal of Respiratory and Critical Care Medicine 2015;191(Meeting Abstracts):A5735. [CENTRAL: 1101168; CRS: 4900132000010003; EMBASE: 72053633]
Yamagata 2016 {published and unpublished data}
-
- AC4115361. A 52‐week, multi‐centre, open‐label study to evaluate the safety and tolerability of GSK573719 125 mcg once‐daily via novel dry powder inhaler (nDPI) in Japanese subjects with chronic obstructive pulmonary disease. gsk‐clinicalstudyregister.com/files2/115361‐Clinical‐Study‐Result‐Summary.pdf (first received 7 August 2012).
-
- NCT01702363. Long‐term safety study for GSK573719 in Japanese (AC4115361) [A 52‐week, multi‐centre, open‐label study to evaluate the safety and tolerability of GSK573719 125 mcg once‐daily via novel dry powder inhaler (nDPI) in Japanese subjects with chronic obstructive pulmonary disease]. clinicaltrials.gov/show/NCT01702363 (first received 20 September 2012). [CRS: 4900132000005788]
-
- Yamagata E, Soutome T, Hashimoto K, Mihara K, Tohda Y. Long‐term (52 weeks) safety and tolerability of umeclidinium in Japanese patients with chronic obstructive pulmonary disease. Current Medical Research and Opinion 2016;32(5):967‐73. [PUBMED: 26782971] - PubMed
Zheng 2015 {published and unpublished data}
-
- DB2114634. A 24‐week randomised, double‐blind and placebo‐controlled study to evaluate the efficacy and safety of GSK573719/GW642444 125/25 mcg and 62.5/25mcg inhalation powder compared with placebo inhalation powder delivered once‐daily via a novel dry powder inhaler in subjects with chronic obstructive pulmonary disease (COPD). gsk‐clinicalstudyregister.com/files/114634/4982/gsk‐114634‐clinical‐study‐re... (first received 16 July 2012).
-
- NCT01636713. A 24‐week study to evaluate the efficacy and safety of GSK573719/GW642444 125/25 mcg and 62.5/25mcg inhalation powder compared with placebo in subjects with COPD [A 24‐week randomised, double‐blind and placebo‐controlled study to evaluate the efficacy and safety of GSK573719/GW642444 125/25 mcg and 62.5/25mcg inhalation powder compared with placebo inhalation powder delivered once‐daily via a novel dry powder inhaler in subjects with chronic obstructive pulmonary disease (COPD)]. clinicaltrials.gov/show/NCT01636713 (first received 5 July 2012). [CRS: 4900132000005773]
-
- Zheng J, Zhong N, Newlands A, Church A, Goh AH. Efficacy and safety of once‐daily inhaled umeclidinium/vilanterol in Asian patients with COPD: results from a randomised, placebo‐controlled study. International Journal of Chronic Obstructive Pulmonary Disease 2015;10:1753‐67. [PUBMED: 26366068] - PMC - PubMed
-
- Zheng JP, Newlands AH, Church A, Goh AH. The efficacy and safety of inhaled umeclidinium bromide/vilanterol in Asian patients with chronic obstructive pulmonary disease. Respirology (Carlton, Vic.) 2014;19(Suppl 3):22. [CENTRAL: 1020039; CRS: 4900126000021800; EMBASE: 71677703]
References to ongoing studies
NCT02184611 {unpublished data only}
-
- AC4117410. A 24 week randomised, double‐blind and placebo controlled study to evaluate the efficacy and safety of 62.5 mcg umeclidinium inhalation powder delivered once daily via a novel dry powder inhaler in subjects with chronic obstructive pulmonary disease. gsk‐clinicalstudyregister.com/study/117410?search=compound&compound=umec... (first received May 2016).
-
- NCT02184611. A 24 week efficacy study of inhaled umeclidinium (UMEC) in patients of chronic obstructive pulmonary disease (COPD) using a novel dry powder inhaler (NDPI) [A 24 week randomised, double‐blind and placebo controlled study to evaluate the efficacy and safety of 62.5 mcg umeclidinium inhalation powder delivered once daily via a novel dry powder inhaler in subjects with chronic obstructive pulmonary disease]. clinicaltrials.gov/show/NCT02184611 (first received 3 July 2014). [CRS: 4900132000005778]
Additional references
ATS/ERS 2011
-
- Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, Molen T, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Annals of Internal Medicine 2011;155(3):179‐91. - PubMed
Bauer 2013
-
- Bauer CM, Morissette MC, Stampfli MR. The influence of cigarette smoking on viral infections: translating bench science to impact COPD pathogenesis and acute exacerbations of COPD clinically. Chest 2013;143(1):196‐206. [PUBMED: 23276842] - PubMed
Buist 2007
-
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD Study): a population‐based prevalence study. Lancet 2007;370(9589):741‐50. [PUBMED: 17765523] - PubMed
Cazzola 2014a
-
- Cazzola M, Matera MG. Triple combinations in chronic obstructive pulmonary disease ‐ is three better than two?. Expert Opinion on Pharmacotherapy 2014; Vol. 15, issue 17:2475‐8. [PUBMED: 25327264] - PubMed
Cazzola 2014b
-
- Cazzola M, Matera MG. Bronchodilators: current and future. Clinics in Chest Medicine 2014;35(1):191‐201. [PUBMED: 24507846] - PubMed
Chapman 2006
-
- Chapman KR, Mannino DM, Soriano JB, Vermeire PA, Buist AS, Thun MJ, et al. Epidemiology and costs of chronic obstructive pulmonary disease. European Respiratory Journal 2006;27(1):188‐207. [PUBMED: 16387952] - PubMed
Cheng 2015
Cosio 2009
-
- Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. New England Journal of Medicine 2009;360(23):2445‐54. [PUBMED: 19494220] - PubMed
Criner 2015
Decramer 2013a
-
- Decramer M, Maltais F, Feldman G, Brooks J, Harris S, Mehta R, et al. Bronchodilation of umeclidinium, a new long‐acting muscarinic antagonist, in COPD patients. Respiratory Physiology and Neurobiology 2013;185(2):393‐9. [PUBMED: 23026438] - PubMed
Donohue 2013a
-
- Donohue JF, Maleki‐Yazdi MR, Kilbride S, Mehta R, Kalberg C, Church A. Efficacy and safety of once‐daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respiratory Medicine 2013;107(10):1538‐46. [PUBMED: 23830094] - PubMed
FDA 2013
-
- FDA approved drug products: Anoro Ellipta. www.accessdata.fda.gov/drugsatfda_docs/label/2013/203975s000lbl.pdf (accessed 15 June 2015).
FDA 2014
-
- FDA approved drug products: Incruse Ellipta. www.accessdata.fda.gov/drugsatfda_docs/label/2014/205382s000lbl.pdf (accessed 5 April 2015).
GOLD 2017
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease (2017 report). http://www.goldcopd.org/ (accessed 4 March 2017).
GRADEproGDT [Computer program]
-
- GRADE Working Group, McMaster University. GRADEproGDT. Version accessed 9 February 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Hagstad 2014
-
- Hagstad S, Bjerg A, Ekerljung L, Backman H, Lindberg A, Ronmark E, et al. Passive smoking exposure is associated with increased risk of COPD in never smokers. Chest 2014;145(6):1298‐304. [PUBMED: 24356778] - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hogg 2009
-
- Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annual Review of Pathology 2009;4:435‐59. [PUBMED: 18954287] - PubMed
Incruse Ellipta
-
- Formulary decision guide: Incruse Ellipta (umeclidinium bromide). https://www.guidelinesinpractice.co.uk/download/pdf/id/226.
Ismaila 2015
Karner 2012
Kelly 2014
-
- Kelly E. Umeclidinium bromide and vilanterol in combination for the treatment of chronic obstructive pulmonary disease. Expert Review of Clinical Pharmacology 2014;7(4):403‐13. [PUBMED: 24909949] - PubMed
Maleki‐Yazdi 2014a
-
- Maleki‐Yazdi MR, Kaelin T, Richard N, Zvarich M, Church A. Efficacy and safety of umeclidinium/vilanterol 62.5/25 mcg and tiotropium 18 mcg in chronic obstructive pulmonary disease: results of a 24‐week, randomised, controlled trial. Respiratory Medicine 2014;108(12):1752‐60. [PUBMED: 25458157] - PubMed
Manickam 2014
-
- Manickam R, Asija A, Aronow WS. Umeclidinium for treating COPD: an evaluation of pharmacologic properties, safety and clinical use. Expert Opinion on Drug Safety 2014;13(11):1555‐61. [PUBMED: 25294427] - PubMed
Mannino 2007
-
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007;370(9589):765‐73. [PUBMED: 17765526] - PubMed
Marchetti 2013
-
- Marchetti N, Criner GJ, Albert RK. Preventing acute exacerbations and hospital admissions in COPD. Chest 2013;143(5):1444‐54. [PUBMED: 23648908] - PubMed
Moher 2009
Ni 2014
Ni 2015
NICE 2010
-
- National Institute for Health and Clinical Excellence. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care (partial update). https://www.nice.org.uk/guidance/cg101/resources/guidance‐chronic‐obstru... (accessed 10 April 2015).
Pleasants 2016
-
- Pleasants RA, Wang T, Gao J, Tang H, Donohue JF. Inhaled umeclidinium in COPD patients: a review and meta‐analysis. Drugs 2016;76(3):343‐61. [PUBMED: 26755180] - PubMed
Prakash 2013
-
- Prakash A, Babu KS, Morjaria JB. Novel anti‐cholinergics in COPD. Drug Discovery Today 2013;18(21‐22):1117‐26. [PUBMED: 23872011] - PubMed
Raherison 2009
-
- Raherison C, Girodet PO. Epidemiology of COPD. European Respiratory Review 2009;18(114):213‐21. [PUBMED: 20956146] - PubMed
Raluy‐Callado 2015
Rennard 2006
-
- Rennard SI, Vestbo J. COPD: the dangerous underestimate of 15%. Lancet 2006;367(9518):1216‐9. [PUBMED: 16631861] - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rivera 2008
-
- Rivera RM, Cosio MG, Ghezzo H, Salazar M, Perez‐Padilla R. Comparison of lung morphology in COPD secondary to cigarette and biomass smoke. International Journal of Tuberculosis and Lung Disease 2008;12(8):972‐7. [PUBMED: 18647460] - PubMed
Rodrigo 2015
-
- Rodrigo GJ, Neffen H. A systematic review of the efficacy and safety of a fixed‐dose combination of umeclidinium and vilanterol for the treatment of COPD. Chest 2015;148(2):397‐407. [PUBMED: 25798635] - PubMed
Salmon 2013
-
- Salmon M, Luttmann MA, Foley JJ, Buckley PT, Schmidt DB, Burman M, et al. Pharmacological characterization of GSK573719 (umeclidinium): a novel, long‐acting, inhaled antagonist of the muscarinic cholinergic receptors for treatment of pulmonary diseases. Journal of Pharmacology and Experimental Therapeutics 2013;345(2):260‐70. [PUBMED: 23435542] - PubMed
Segreti 2014
-
- Segreti A, Calzetta L, Rogliani P, Cazzola M. Umeclidinium for the treatment of chronic obstructive pulmonary disease. Expert Review of Respiratory Medicine 2014;8(6):665‐71. [PUBMED: 25312239] - PubMed
Spyratos 2015
Sutherland 2004
-
- Sutherland ER, Cherniack RM. Management of chronic obstructive pulmonary disease. New England Journal of Medicine 2004;350(26):2689‐97. [PUBMED: 15215485] - PubMed
TSANZ 2014
-
- Abramson M, Crockett AJ, Dabscheck E, Frith PA, George J, Glasgow N, et al. The COPD‐X Plan: Australian and New Zealand guidelines for the management of chronic obstructive pulmonary disease. Version 2.39, October 2014. http://www.copdx.org.au/ (accessed 10 April 2015).
Vestbo 2011
-
- Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, et al. Changes in forced expiratory volume in 1 second over time in COPD. New England Journal of Medicine 2011;365(13):1184‐92. - PubMed
WHO 2015
-
- World Health Organization. Chronic obstructive pulmonary disease (COPD). who.int/mediacentre/factsheets/fs315/en/ (accessed 30 May 2015).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

