Plastid thylakoid architecture optimizes photosynthesis in diatoms
- PMID: 28631733
- PMCID: PMC5481826
- DOI: 10.1038/ncomms15885
Plastid thylakoid architecture optimizes photosynthesis in diatoms
Abstract
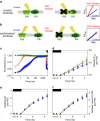
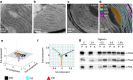
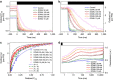
Photosynthesis is a unique process that allows independent colonization of the land by plants and of the oceans by phytoplankton. Although the photosynthesis process is well understood in plants, we are still unlocking the mechanisms evolved by phytoplankton to achieve extremely efficient photosynthesis. Here, we combine biochemical, structural and in vivo physiological studies to unravel the structure of the plastid in diatoms, prominent marine eukaryotes. Biochemical and immunolocalization analyses reveal segregation of photosynthetic complexes in the loosely stacked thylakoid membranes typical of diatoms. Separation of photosystems within subdomains minimizes their physical contacts, as required for improved light utilization. Chloroplast 3D reconstruction and in vivo spectroscopy show that these subdomains are interconnected, ensuring fast equilibration of electron carriers for efficient optimum photosynthesis. Thus, diatoms and plants have converged towards a similar functional distribution of the photosystems although via different thylakoid architectures, which likely evolved independently in the land and the ocean.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Dekker J. P. & Boekema E. J. Supramolecular organization of thylakoid membrane proteins in green plants. Biochim. Biophys. Acta 1706, 12–39 (2005). - PubMed
-
- Kirchhoff H., Schöttler M.-A., Maurer J. & Weis E. Plastocyanin redox kinetics in spinach chloroplasts: evidence for disequilibrium in the high potential chain. Biochim. Biophys. Acta 1659, 63–72 (2004). - PubMed
-
- Bedoshvili Y. D., Popkova T. P. & Likhoshway Y. V. Chloroplast structure of diatoms of different classes. Cell Tissue Biol. 3, 297–310 (2009).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

