Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma
- PMID: 28633365
- PMCID: PMC5834070
- DOI: 10.1093/annonc/mdx289
Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma
Abstract
Background: Copanlisib is a pan-class I phosphatidylinositol 3-kinase inhibitor with predominant activity against the α- and δ-isoforms.
Patients and methods: This phase II study evaluated the response rate of copanlisib administered intravenously on days 1, 8, and 15 of a 28-day cycle, in patients with indolent or aggressive malignant lymphoma. Archival tumor tissues were used for immunohistochemistry, gene-expression profiling, and mutation analysis.
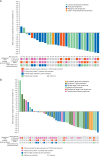
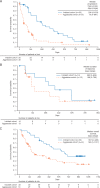
Results: Thirty-three patients with indolent lymphoma and 51 with aggressive lymphoma received copanlisib. Follicular lymphoma (48.5%) and peripheral T-cell lymphoma (33.3%) were the most common histologic subtypes. Most patients (78.6%) had received prior rituximab and 54.8% were rituximab-refractory. Median duration of treatment was 23 and 8 weeks in the indolent and aggressive cohorts, respectively (overall range 2-138). Eighty patients were evaluated for efficacy. The objective response rate was 43.7% (14/32) in the indolent cohort and 27.1% (13/48) in the aggressive cohort; median progression-free survival was 294 days (range 0-874) and 70 days (range 0-897), respectively; median duration of response was 390 days (range 0-825) and 166 days (range 0-786), respectively. Common adverse events included hyperglycemia (57.1%; grade ≥3, 23.8%), hypertension (54.8%; grade ≥3, 40.5%), and diarrhea (40.5%; grade ≥3, 4.8%), all generally manageable. Neutropenia occurred in 28.6% of patients (grade 4, 11.9%). Molecular analyses showed enhanced antitumor activity in tumors with upregulated phosphatidylinositol 3-kinase pathway gene expression.
Conclusion: Intravenous copanlisib demonstrated promising efficacy and manageable toxicity in heavily pretreated patients with various subtypes of indolent and aggressive malignant lymphoma. Subtype-specific studies of copanlisib in patients with follicular, peripheral T-cell, and mantle cell lymphomas are ongoing. This trial is registered with ClinicalTrials.gov number NCT01660451 (Part A).
Keywords: PI3K inhibitor; copanlisib; malignant lymphoma; treatment.
© The Author 2017. Published by Oxford University Press on behalf of the European Society for Medical Oncology.
Figures
Comment in
-
Revival of PI3K inhibitors in non-Hodgkin's lymphoma.Ann Oncol. 2017 Sep 1;28(9):2047-2049. doi: 10.1093/annonc/mdx392. Ann Oncol. 2017. PMID: 28911078 No abstract available.
References
-
- de Claro RA, McGinn KM, Verdun N. et al. FDA approval: ibrutinib for patients with previously treated mantle cell lymphoma and previously treated chronic lymphocytic leukemia. Clin Cancer Res 2015; 21: 3586–3590. - PubMed
-
- Miller BW, Przepiorka D, de Claro RA. et al. FDA approval: idelalisib monotherapy for the treatment of patients with follicular lymphoma and small lymphocytic lymphoma. Clin Cancer Res 2015; 21: 1525–1529. - PubMed
-
- Center for Drug Evaluation and Research. Application number: 206545Orig1s000. Medical Review(s). Clinical Review. Zydelig® (Idelalisib). http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/206545Orig1s000Me... (20 March 2017, date last accessed).
-
- Pongas G, Cheson BD.. PI3K signaling pathway in normal B cells and indolent B-cell malignancies. Semin Oncol 2016; 43: 647–654. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

