Digenic DUOX1 and DUOX2 Mutations in Cases With Congenital Hypothyroidism
- PMID: 28633507
- PMCID: PMC5587079
- DOI: 10.1210/jc.2017-00529
Digenic DUOX1 and DUOX2 Mutations in Cases With Congenital Hypothyroidism
Abstract
Context: The DUOX2 enzyme generates hydrogen peroxide (H2O2), a crucial electron acceptor for the thyroid peroxidase-catalyzed iodination and coupling reactions mediating thyroid hormone biosynthesis. DUOX2 mutations result in dyshormonogenetic congenital hypothyroidism (CH) that may be phenotypically heterogeneous, leading to the hypothesis that CH severity may be influenced by environmental factors (e.g., dietary iodine) and oligogenic modifiers (e.g., variants in the homologous reduced form of NAD phosphate-oxidase DUOX1). However, loss-of-function mutations in DUOX1 have not hitherto been described, and its role in thyroid biology remains undefined.
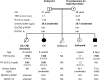
Case description: We previously described a Proband and her brother (P1, P2) with unusually severe CH associated with a DUOX2 homozygous nonsense mutation (p.R434*); P1, P2: thyrotropin >100 µU/mL [reference range (RR) 0.5 to 6.3]; and P1: free T4 (FT4) <0.09 ng/dL (RR 0.9 to 2.3). Subsequent studies have revealed a homozygous DUOX1 mutation (c.1823-1G>C) resulting in aberrant splicing and a protein truncation (p.Val607Aspfs*43), which segregates with CH in this kindred.
Conclusion: This is a report of digenic mutations in DUOX1 and DUOX2 in association with CH, and we hypothesize that the inability of DUOX1 to compensate for DUOX2 deficiency in this kindred may underlie the severe CH phenotype. Our studies provide evidence for a digenic basis for CH and support the notion that oligogenicity as well as environmental modulators may underlie phenotypic variability in genetically ascertained CH.
Copyright © 2017 Endocrine Society
Figures

Similar articles
-
New phenotypes in thyroid dyshormonogenesis: hypothyroidism due to DUOX2 mutations.Endocr Dev. 2007;10:99-117. doi: 10.1159/000106822. Endocr Dev. 2007. PMID: 17684392 Review.
-
Identification and analyzes of DUOX2 mutations in two familial congenital hypothyroidism cases.Endocrine. 2021 Apr;72(1):147-156. doi: 10.1007/s12020-020-02437-8. Epub 2020 Aug 15. Endocrine. 2021. PMID: 32803677
-
DUOX Defects and Their Roles in Congenital Hypothyroidism.Methods Mol Biol. 2019;1982:667-693. doi: 10.1007/978-1-4939-9424-3_37. Methods Mol Biol. 2019. PMID: 31172499 Review.
-
Conformation of the N-Terminal Ectodomain Elicits Different Effects on DUOX Function: A Potential Impact on Congenital Hypothyroidism Caused by a H2O2 Production Defect.Thyroid. 2018 Aug;28(8):1052-1062. doi: 10.1089/thy.2017.0596. Epub 2018 Jul 24. Thyroid. 2018. PMID: 29845893
-
Correlation of DUOX2 residual enzymatic activity with phenotype in congenital hypothyroidism caused by biallelic DUOX2 defects.Clin Genet. 2021 Dec;100(6):713-721. doi: 10.1111/cge.14065. Epub 2021 Sep 29. Clin Genet. 2021. PMID: 34564849
Cited by
-
New genetics in congenital hypothyroidism.Endocrine. 2021 Mar;71(3):696-705. doi: 10.1007/s12020-021-02646-9. Epub 2021 Mar 1. Endocrine. 2021. PMID: 33650047 Review.
-
DUOX2 and DUOXA2 Variants Confer Susceptibility to Thyroid Dysgenesis and Gland-in-situ With Congenital Hypothyroidism.Front Endocrinol (Lausanne). 2020 Apr 21;11:237. doi: 10.3389/fendo.2020.00237. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32425884 Free PMC article.
-
Genotype and phenotype correlation in a cohort of Chinese congenital hypothyroidism patients with DUOX2 mutations.Ann Transl Med. 2020 Dec;8(24):1649. doi: 10.21037/atm-20-7165. Ann Transl Med. 2020. PMID: 33490161 Free PMC article.
-
MECHANISMS IN ENDOCRINOLOGY: The pathophysiology of transient congenital hypothyroidism.Eur J Endocrinol. 2022 Jun 20;187(2):R1-R16. doi: 10.1530/EJE-21-1278. Eur J Endocrinol. 2022. PMID: 35588090 Free PMC article. Review.
-
Thyroid Gene Mutations in Pregnant and Breastfeeding Women Diagnosed With Transient Congenital Hypothyroidism: Implications for the Offspring's Health.Front Endocrinol (Lausanne). 2021 Oct 14;12:679002. doi: 10.3389/fendo.2021.679002. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34721286 Free PMC article. Review.
References
-
- Muzza M, Rabbiosi S, Vigone MC, Zamproni I, Cirello V, Maffini MA, Maruca K, Schoenmakers N, Beccaria L, Gallo F, Park SM, Beck-Peccoz P, Persani L, Weber G, Fugazzola L. The clinical and molecular characterization of patients with dyshormonogenic congenital hypothyroidism reveals specific diagnostic clues for DUOX2 defects. J Clin Endocrinol Metab. 2014;99(3):E544–E553. - PubMed
-
- Cangul H, Aycan Z, Kendall M, Bas VN, Saglam Y, Barrett TG, Maher ER. A truncating DUOX2 mutation (R434X) causes severe congenital hypothyroidism. J Pediatr Endocrinol Metab. 2014;27(3-4):323–327. - PubMed
-
- Moreno JC, Bikker H, Kempers MJE, van Trotsenburg ASP, Baas F, de Vijlder JJM, Vulsma T, Ris-Stalpers C. Inactivating mutations in the gene for thyroid oxidase 2 (THOX2) and congenital hypothyroidism. N Engl J Med. 2002;347(2):95–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

