Restoration of CFTR Activity in Ducts Rescues Acinar Cell Function and Reduces Inflammation in Pancreatic and Salivary Glands of Mice
- PMID: 28634110
- PMCID: PMC5623154
- DOI: 10.1053/j.gastro.2017.06.011
Restoration of CFTR Activity in Ducts Rescues Acinar Cell Function and Reduces Inflammation in Pancreatic and Salivary Glands of Mice
Abstract
Background & aims: Sjögren's syndrome and autoimmune pancreatitis are disorders with decreased function of salivary, lacrimal glands, and the exocrine pancreas. Nonobese diabetic/ShiLTJ mice and mice transduced with the cytokine BMP6 develop Sjögren's syndrome and chronic pancreatitis and MRL/Mp mice are models of autoimmune pancreatitis. Cystic fibrosis transmembrane conductance regulator (CFTR) is a ductal Cl- channel essential for ductal fluid and HCO3- secretion. We used these models to ask the following questions: is CFTR expression altered in these diseases, does correction of CFTR correct gland function, and most notably, does correcting ductal function correct acinar function?
Methods: We treated the mice models with the CFTR corrector C18 and the potentiator VX770. Glandular, ductal, and acinar cells damage, infiltration, immune cells and function were measured in vivo and in isolated duct/acini.
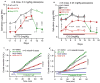
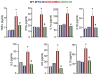
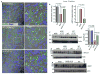
Results: In the disease models, CFTR expression is markedly reduced. The salivary glands and pancreas are inflamed with increased fibrosis and tissue damage. Treatment with VX770 and, in particular, C18 restored salivation, rescued CFTR expression and localization, and nearly eliminated the inflammation and tissue damage. Transgenic overexpression of CFTR exclusively in the duct had similar effects. Most notably, the markedly reduced acinar cell Ca2+ signaling, Orai1, inositol triphosphate receptors, Aquaporin 5 expression, and fluid secretion were restored by rescuing ductal CFTR.
Conclusions: Our findings reveal that correcting ductal function is sufficient to rescue acinar cell function and suggests that CFTR correctors are strong candidates for the treatment of Sjögren's syndrome and pancreatitis.
Keywords: CFTR; Duct; Pancreatitis; Sjögren's syndrome.
Copyright © 2017 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures




References
-
- Melvin JE, Yule D, Shuttleworth T, et al. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–69. - PubMed
-
- Buchwald M. Cystic fibrosis: from the gene to the dream. Clin Invest Med. 1996;19:304–10. - PubMed
-
- Lerch MM, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology. 2013;144:1180–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

