Ascorbate protects the diheme enzyme, MauG, against self-inflicted oxidative damage by an unusual antioxidant mechanism
- PMID: 28634178
- PMCID: PMC5862032
- DOI: 10.1042/BCJ20170349
Ascorbate protects the diheme enzyme, MauG, against self-inflicted oxidative damage by an unusual antioxidant mechanism
Abstract
Ascorbate protects MauG from self-inactivation that occurs during the autoreduction of the reactive bis-FeIV state of its diheme cofactor. The mechanism of protection does not involve direct reaction with reactive oxygen species in solution. Instead, it binds to MauG and mitigates oxidative damage that occurs via internal transfer of electrons from amino acid residues within the protein to the high-valent hemes. The presence of ascorbate does not inhibit the natural catalytic reaction of MauG, which catalyzes oxidative post-translational modifications of a substrate protein that binds to the surface of MauG and is oxidized by the high-valent hemes via long-range electron transfer. Ascorbate was also shown to prolong the activity of a P107V MauG variant that is more prone to inactivation. A previously unknown ascorbate peroxidase activity of MauG was characterized with a kcat of 0.24 s-1 and a Km of 2.2 µM for ascorbate. A putative binding site for ascorbate was inferred from inspection of the crystal structure of MauG and comparison with the structure of soybean ascorbate peroxidase with bound ascorbate. The ascorbate bound to MauG was shown to accelerate the rates of both electron transfers to the hemes and proton transfers to hemes which occur during the multistep autoreduction to the diferric state which is accompanied by oxidative damage. A structural basis for these effects is inferred from the putative ascorbate-binding site. This could be a previously unrecognized mechanism by which ascorbate mitigates oxidative damage to heme-dependent enzymes and redox proteins in nature.
Keywords: antioxidants; cytochrome; electron transfer; oxidative stress; peroxidases; proton transfer.
© 2017 The Author(s); published by Portland Press Limited on behalf of the Biochemical Society.
Conflict of interest statement
The authors do not have any conflicting financial interests to declare.
Figures

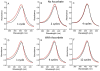


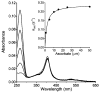

References
-
- Wang Y, Graichen ME, Liu A, Pearson AR, Wilmot CW, Davidson VL. MauG, a novel diheme protein required for tryptophan tryptophylquinone biogenesis. Biochemistry. 2003;42:7318–7325. - PubMed
-
- Pearson AR, de la Mora-Rey T, Graichen ME, Wang Y, Jones LH, Marimanikkupam S, Aggar SA, Grimsrud PA, Davidson VL, Wilmot CW. Further insights into quinone cofactor biogenesis: Probing the role of MauG in methylamine dehydrogenase TTQ formation. Biochemistry. 2004;43:5494–5502. - PubMed
-
- Davidson VL. Pyrroloquinoline quinone (PQQ) from methanol dehydrogenase and tryptophan tryptophylquinone (TTQ) from methylamine dehydrogenase. Adv Protein Chem. 2001;58:95–140. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

