The effect of sustained virological response on the risk of extrahepatic manifestations of hepatitis C virus infection
- PMID: 28634198
- PMCID: PMC6292199
- DOI: 10.1136/gutjnl-2017-313983
The effect of sustained virological response on the risk of extrahepatic manifestations of hepatitis C virus infection
Abstract
Background and aim: Chronic HCV infection is associated with several extrahepatic manifestations (EHMs). Data on the effect of sustained virological response (SVR) on the risk of EHMs are limited.
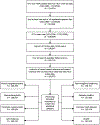
Methods: We conducted a retrospective cohort study using data of patients from the US Veterans Affairs HCV Clinical Case Registry who had a positive HCV RNA test (10/1999-08/2009). Patients receiving interferon-based antiviral therapy (AVT) were identified. SVR was defined as negative HCV RNA at least 12 weeks after end of AVT. Risks of eight incident EHMs were evaluated in Cox regression models.
Results: Of the 160 875 HCV-infected veterans, 31 143 (19.4%) received AVT, of whom 10 575 (33.9%) experienced SVR. EHM risk was reduced in the SVR group compared with untreated patients for mixed cryoglobulinaemia (adjusted HR (aHR)=0.61; 95% CI 0.39 to 0.94), glomerulonephritis (aHR=0.62; 95% CI 0.48 to 0.79), porphyria cutanea tarda (PCT) (aHR=0.41; 95% CI 0.20 to 0.83), non-Hodgkin's lymphoma (NHL) (aHR=0.64; 95% CI 0.43 to 0.95), diabetes (aHR=0.82; 95% CI 0.76 to 0.88) and stroke (aHR=0.84; 95% CI 0.74 to 0.94), but not for lichen planus (aHR=1.11; 95% CI 0.78 to 1.56) or coronary heart disease (aHR=1.12; 95% CI 0.81 to 1.56). Risk reductions were also observed when patients with SVR were compared with treated patients without SVR for mixed cryoglobulinaemia, glomerulonephritis, PCT and diabetes. Significant reductions in the magnitude of aHRs towards the null with increasing time to initiation of AVT after HCV diagnosis were observed for glomerulonephritis, NHL and stroke.
Conclusions: Risks of several EHMs of HCV infection are reduced after AVT with SVR. However, early initiation of AVT may be required to reduce the risk of glomerulonephritis, NHL and stroke.
Keywords: Hepatitis C virus; antiviral therapy; extrahepatic manifestations; sustained virological response.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: HAT is a consultant for Gilead Sciences, Janssen Pharmaceuticals, Merck and Co., Dynavax Technologies, Vertex Pharmaceuticals, and Genentech, and has received research grants from Gilead Sciences, Merck and Co., and Vertex Pharmaceuticals. Other authors have no conflicts of interest to declare.
Figures

Comment in
-
Can we prevent and modify cardiometabolic disorders by controlling HCV infection?Gut. 2018 Mar;67(3):403-404. doi: 10.1136/gutjnl-2017-314505. Epub 2017 Jul 13. Gut. 2018. PMID: 28706079 No abstract available.
References
-
- Mohd Hanafiah K, Groeger J, Flaxman AD, et al. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013;57:1333–42. - PubMed
-
- Spiegel BM, Younossi ZM, Hays RD, et al. Impact of hepatitis C on health related quality of life: a systematic review and quantitative assessment. Hepatology 2005;41:790–800. - PubMed
-
- Negro F, Forton D, Craxi A, et al. Extrahepatic Morbidity and Mortality of Chronic Hepatitis C. Gastroenterology 2015;149:1345–60. - PubMed
-
- Ferri C, Ramos-Casals M, Zignego AL, et al. International diagnostic guidelines for patients with HCV-related extrahepatic manifestations. A multidisciplinary expert statement. Autoimmun Rev 2016;15:1145–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
