Prolonged darkness reduces liver fibrosis in a mouse model of primary sclerosing cholangitis by miR-200b down-regulation
- PMID: 28634212
- PMCID: PMC5987749
- DOI: 10.1096/fj.201700097R
Prolonged darkness reduces liver fibrosis in a mouse model of primary sclerosing cholangitis by miR-200b down-regulation
Abstract
Melatonin therapy or prolonged exposure to complete darkness reduces biliary hyperplasia and liver fibrosis in bile-duct-ligated (BDL) rats; however, no information exists in primary sclerosing cholangitis (PSC). Thus, we aimed to determine the therapeutic effects of prolonged dark therapy or melatonin administration on hepatic fibrosis in the multidrug resistance gene 2-knockout (Mdr2-/-) mouse model of PSC. Melatonin levels, biliary mass, liver fibrosis, angiogenesis and miR-200b expression were evaluated in wild-type and Mdr2-/- mice exposed to darkness or melatonin treatment or in male patients with PSC and healthy controls. Mdr2-/- mice were also treated with miR-200b inhibitor or control before evaluating biliary mass, liver fibrosis, and angiogenesis. After overexpression of arylalkylamine N-acetyltransferase (AANAT; the enzyme regulating melatonin synthesis) or inhibition of miR-200b in cholangiocytes and hepatic stellate cells in vitro, we evaluated angiogenesis and fibrosis gene expression. After exposure to darkness or administration of melatonin, Mdr2-/- mice show elevated serum melatonin levels and inhibition of biliary mass, along with reduction of liver fibrosis and angiogenesis. MicroRNA PCR analysis demonstrated that miR-200b expression increased in Mdr2-/- mice and patients with PSC compared with controls and decreased in Mdr2-/- mice subjected to dark exposure or melatonin treatment. Inhibition of miR-200b in Mdr2-/- ablates biliary proliferation, liver fibrosis, and angiogenesis. In vitro, overexpression of AANAT or inhibition of miR-200b in cholangiocytes and hepatic stellate cells decreased the expression of miR-200b, angiogenesis, and fibrosis genes. Dark therapy or targeting melatonin/miR-200b axis may be important in the management of biliary damage and liver fibrosis in cholangiopathies including PSC.-Wu, N., Meng, F., Zhou, T., Han, Y., Kennedy, L., Venter, J., Francis, H., DeMorrow, S., Onori, P., Invernizzi, P., Bernuzzi, F., Mancinelli, R., Gaudio, E., Franchitto, A., Glaser, S., Alpini G. Prolonged darkness reduces liver fibrosis in a mouse model of primary sclerosing cholangitis by miR-200b down-regulation.
Keywords: angiogenesis; biliary epithelium; cholangiopathy; cholestasis; miRNA.
© FASEB.
Conflict of interest statement
This work was supported by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology (Baylor Scott & White), a Translational Genomics Research Institute (TGEN) seed grant, and a Veterans Affairs (VA) Research Career Scientist Award (to G.A.); and VA Merit Awards 5I01BX000574 (to G.A.) 5I01BX002192 (to S.G.), 1I01BX001724 (to F.M.), 1I01BX003031 (to H.F.), and 1IO1BX002638 (to S.D.); University of Rome “La Sapienza,” and Fondo per gli Investimenti della Ricerca di Base (FIRB) Accordi di Programma 2010–RBAP10Z7FS (to E.G.); U.S. National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases Grants DK054811, DK076898, DK107310, DK110035, and DK062975 (to G.A., F.M., and S.G.), DK108959 (to H.F.), and DK082435 to (S.D., N.W., and F.M.). This publication is the result of work supported by resources at the Central Texas Veterans Health Care System. The content is the responsibility of the authors alone, and does not necessarily reflect the views or policies of the Department of Veterans Affairs or the U.S. Government. G.A., A.F., and S.G. share senior authorship. The authors declare no conflicts of interest.
Figures
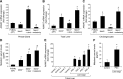
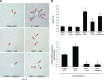
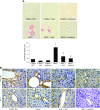


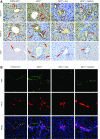


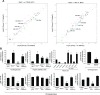
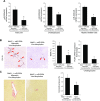



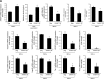


References
-
- Melum E., Franke A., Schramm C., Weismüller T. J., Gotthardt D. N., Offner F. A., Juran B. D., Laerdahl J. K., Labi V., Björnsson E., Weersma R. K., Henckaerts L., Teufel A., Rust C., Ellinghaus E., Balschun T., Boberg K. M., Ellinghaus D., Bergquist A., Sauer P., Ryu E., Hov J. R., Wedemeyer J., Lindkvist B., Wittig M., Porte R. J., Holm K., Gieger C., Wichmann H. E., Stokkers P., Ponsioen C. Y., Runz H., Stiehl A., Wijmenga C., Sterneck M., Vermeire S., Beuers U., Villunger A., Schrumpf E., Lazaridis K. N., Manns M. P., Schreiber S., and Karlsen T. H. (2011) Genome-wide association analysis in primary sclerosing cholangitis identifies two non-HLA susceptibility loci. Nat. Genet. 43, 17–19 - PMC - PubMed
-
- Fickert P., Fuchsbichler A., Wagner M., Zollner G., Kaser A., Tilg H., Krause R., Lammert F., Langner C., Zatloukal K., Marschall H. U., Denk H., and Trauner M. (2004) Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology 127, 261–274 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK108959/DK/NIDDK NIH HHS/United States
- R21 AA025157/AA/NIAAA NIH HHS/United States
- R21 AA025997/AA/NIAAA NIH HHS/United States
- R01 DK062975/DK/NIDDK NIH HHS/United States
- R01 DK110035/DK/NIDDK NIH HHS/United States
- R01 DK054811/DK/NIDDK NIH HHS/United States
- I01 BX002192/BX/BLRD VA/United States
- R01 DK107310/DK/NIDDK NIH HHS/United States
- R01 DK082435/DK/NIDDK NIH HHS/United States
- I01 BX000574/BX/BLRD VA/United States
- I01 BX001724/BX/BLRD VA/United States
- R01 DK076898/DK/NIDDK NIH HHS/United States
- I01 BX003031/BX/BLRD VA/United States
- I01 BX002638/BX/BLRD VA/United States
- R01 DK115184/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

