Vaccination with High-Affinity Epitopes Impairs Antitumor Efficacy by Increasing PD-1 Expression on CD8+ T Cells
- PMID: 28634215
- PMCID: PMC5821110
- DOI: 10.1158/2326-6066.CIR-16-0374
Vaccination with High-Affinity Epitopes Impairs Antitumor Efficacy by Increasing PD-1 Expression on CD8+ T Cells
Abstract
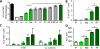
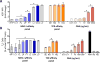
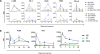
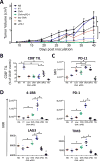
Antitumor vaccines encoding self-antigens generally have low immunogenicity in clinical trials. Several approaches are aimed at improving vaccine immunogenicity, including efforts to alter encoded epitopes. Immunization with epitopes altered for increased affinity for the major histocompatibility complex (MHC) or T-cell receptor (TCR) elicits greater numbers of CD8 T cells but inferior antitumor responses. Our previous results suggested that programmed death 1 (PD-1) and its ligand (PD-L1) increased on antigen-specific CD8 T cells and tumor cells, respectively, after high-affinity vaccination. In this report, we use two murine models to investigate whether the dose, MHC affinity, or TCR affinity of an epitope affected the antitumor response via the PD-1/PD-L1 axis. T cells activated with high-affinity epitopes resulted in prolonged APC:T-cell contact time that led to elevated, persistent PD-1 expression, and expression of other checkpoint molecules, in vitro and in vivo Immunization with high-affinity epitopes also decreased antitumor efficacy in the absence of PD-1 blockade. Thus, APC:T-cell contact time can be altered by epitope affinity and lead to therapeutically relevant changes in vaccine efficacy mediated by changes in PD-1 expression. These findings have implications for the use of agents targeting PD-1 expression or function whenever high-affinity CD8 T cells are elicited or supplied by means of vaccination or adoptive transfer. Cancer Immunol Res; 5(8); 630-41. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures


References
-
- Mattarollo SR, Frazer IH, Leggatt GR. Regulation of immune responses to HPVinfection and during HPV- directed immunotherapy. 2011;239:85–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

