Representations of Time-Varying Cochlear Implant Stimulation in Auditory Cortex of Awake Marmosets (Callithrix jacchus)
- PMID: 28634306
- PMCID: PMC5518426
- DOI: 10.1523/JNEUROSCI.0093-17.2017
Representations of Time-Varying Cochlear Implant Stimulation in Auditory Cortex of Awake Marmosets (Callithrix jacchus)
Abstract
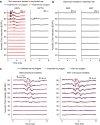
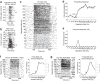
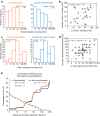
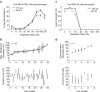
Electrical stimulation of the auditory periphery organ by cochlear implant (CI) generates highly synchronized inputs to the auditory system. It has long been thought such inputs would lead to highly synchronized neural firing along the ascending auditory pathway. However, neurophysiological studies with hearing animals have shown that the central auditory system progressively converts temporal representations of time-varying sounds to firing rate-based representations. It is not clear whether this coding principle also applies to highly synchronized CI inputs. Higher-frequency modulations in CI stimulation have been found to evoke largely transient responses with little sustained firing in previous studies of the primary auditory cortex (A1) in anesthetized animals. Here, we show that, in addition to neurons displaying synchronized firing to CI stimuli, a large population of A1 neurons in awake marmosets (Callithrix jacchus) responded to rapid time-varying CI stimulation with discharges that were not synchronized to CI stimuli, yet reflected changing repetition frequency by increased firing rate. Marmosets of both sexes were included in this study. By comparing directly each neuron's responses to time-varying acoustic and CI signals, we found that individual A1 neurons encode both modalities with similar firing patterns (stimulus-synchronized or nonsynchronized). These findings suggest that A1 neurons use the same basic coding schemes to represent time-varying acoustic or CI stimulation and provide new insights into mechanisms underlying how the brain processes natural sounds via a CI device.SIGNIFICANCE STATEMENT In modern cochlear implant (CI) processors, the temporal information in speech or environmental sounds is delivered through modulated electric pulse trains. How the auditory cortex represents temporally modulated CI stimulation across multiple time scales has remained largely unclear. In this study, we compared directly neuronal responses in primary auditory cortex (A1) to time-varying acoustic and CI signals in awake marmoset monkeys (Callithrix jacchus). We found that A1 neurons encode both modalities using similar coding schemes, but some important differences were identified. Our results provide insights into mechanisms underlying how the brain processes sounds via a CI device and suggest a candidate neural code underlying rate-pitch perception limitations often observed in CI users.
Keywords: auditory cortex; cochlear implant; marmoset.
Copyright © 2017 the authors 0270-6474/17/377008-15$15.00/0.
Figures






Comment in
-
Does Auditory Cortex Code Temporal Information from Acoustic and Cochlear Implant Stimulation in a Similar Way?J Neurosci. 2018 Jan 10;38(2):260-262. doi: 10.1523/JNEUROSCI.2774-17.2017. J Neurosci. 2018. PMID: 29321145 Free PMC article. No abstract available.
Similar articles
-
Neural representations of sinusoidal amplitude and frequency modulations in the primary auditory cortex of awake primates.J Neurophysiol. 2002 May;87(5):2237-61. doi: 10.1152/jn.2002.87.5.2237. J Neurophysiol. 2002. PMID: 11976364
-
Representation of a species-specific vocalization in the primary auditory cortex of the common marmoset: temporal and spectral characteristics.J Neurophysiol. 1995 Dec;74(6):2685-706. doi: 10.1152/jn.1995.74.6.2685. J Neurophysiol. 1995. PMID: 8747224
-
Selective Neuronal Activation by Cochlear Implant Stimulation in Auditory Cortex of Awake Primate.J Neurosci. 2016 Dec 7;36(49):12468-12484. doi: 10.1523/JNEUROSCI.1699-16.2016. J Neurosci. 2016. PMID: 27927962 Free PMC article.
-
Neural coding strategies in auditory cortex.Hear Res. 2007 Jul;229(1-2):81-93. doi: 10.1016/j.heares.2007.01.019. Epub 2007 Jan 25. Hear Res. 2007. PMID: 17346911 Review.
-
Neural mechanisms for the abstraction and use of pitch information in auditory cortex.J Neurosci. 2012 Sep 26;32(39):13339-42. doi: 10.1523/JNEUROSCI.3814-12.2012. J Neurosci. 2012. PMID: 23015423 Free PMC article. Review.
Cited by
-
Does Auditory Cortex Code Temporal Information from Acoustic and Cochlear Implant Stimulation in a Similar Way?J Neurosci. 2018 Jan 10;38(2):260-262. doi: 10.1523/JNEUROSCI.2774-17.2017. J Neurosci. 2018. PMID: 29321145 Free PMC article. No abstract available.
-
Improved Neural Coding of ITD with Bilateral Cochlear Implants by Introducing Short Inter-pulse Intervals.J Assoc Res Otolaryngol. 2018 Dec;19(6):681-702. doi: 10.1007/s10162-018-00693-0. Epub 2018 Sep 6. J Assoc Res Otolaryngol. 2018. PMID: 30191423 Free PMC article.
-
The Perception of Multiple Simultaneous Pitches as a Function of Number of Spectral Channels and Spectral Spread in a Noise-Excited Envelope Vocoder.J Assoc Res Otolaryngol. 2020 Feb;21(1):61-72. doi: 10.1007/s10162-019-00738-y. Epub 2020 Feb 11. J Assoc Res Otolaryngol. 2020. PMID: 32048077 Free PMC article.
-
Auditory cortical plasticity in cochlear implant users.Curr Opin Neurobiol. 2020 Feb;60:108-114. doi: 10.1016/j.conb.2019.11.003. Epub 2019 Dec 18. Curr Opin Neurobiol. 2020. PMID: 31864104 Free PMC article. Review.
-
The Common Marmoset as a Novel Non-human Primate Model for Inner Ear Research.JMA J. 2025 Jul 15;8(3):679-688. doi: 10.31662/jmaj.2025-0142. Epub 2025 May 30. JMA J. 2025. PMID: 40786498 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
