Prefrontal Neurons Encode a Solution to the Credit-Assignment Problem
- PMID: 28634307
- PMCID: PMC5518425
- DOI: 10.1523/JNEUROSCI.3311-16.2017
Prefrontal Neurons Encode a Solution to the Credit-Assignment Problem
Abstract
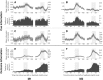
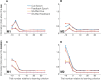
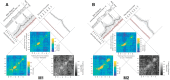
To adapt successfully to our environments, we must use the outcomes of our choices to guide future behavior. Critically, we must be able to correctly assign credit for any particular outcome to the causal features which preceded it. In some cases, the causal features may be immediately evident, whereas in others they may be separated in time or intermingled with irrelevant environmental stimuli, creating a potentially nontrivial credit-assignment problem. We examined the neuronal representation of information relevant for credit assignment in the dorsolateral prefrontal cortex (dlPFC) of two male rhesus macaques performing a task that elicited key aspects of this problem. We found that neurons conveyed the information necessary for credit assignment. Specifically, neuronal activity reflected both the relevant cues and outcomes at the time of feedback and did so in a manner that was stable over time, in contrast to prior reports of representational instability in the dlPFC. Furthermore, these representations were most stable early in learning, when credit assignment was most needed. When the same features were not needed for credit assignment, these neuronal representations were much weaker or absent. These results demonstrate that the activity of dlPFC neurons conforms to the basic requirements of a system that performs credit assignment, and that spiking activity can serve as a stable mechanism that links causes and effects.SIGNIFICANCE STATEMENT Credit assignment is the process by which we infer the causes of our successes and failures. We found that neuronal activity in the dorsolateral prefrontal cortex conveyed the necessary information for performing credit assignment. Importantly, while there are various potential mechanisms to retain a "trace" of the causal events over time, we observed that spiking activity was sufficiently stable to act as the link between causes and effects, in contrast to prior reports that suggested spiking representations were unstable over time. In addition, we observed that this stability varied as a function of learning, such that the neural code was more reliable over time during early learning, when it was most needed.
Keywords: credit assignment; learning; monkey; population coding; prefrontal cortex; single neuron.
Copyright © 2017 the authors 0270-6474/17/376995-13$15.00/0.
Figures







References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
