Infections of horses and shrews with Bornaviruses in Upper Austria: a novel endemic area of Borna disease
- PMID: 28634359
- PMCID: PMC5520313
- DOI: 10.1038/emi.2017.36
Infections of horses and shrews with Bornaviruses in Upper Austria: a novel endemic area of Borna disease
Abstract
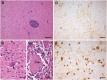
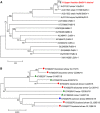
Borna disease, a lethal infection with Borna disease virus-1 (BoDV-1), was diagnosed in four horses from Upper Austria in 2015 and 2016. All cases occurred in winter (two cases in February 2015 and two cases in December 2016), and the maximal distance of the affected stables was 17 km. To demonstrate whether the causative agent was also harbored by its reservoir host, the bicolored white-toothed shrew (Crocidura leucodon), 28 shrews from this geographic area were collected in 2015 and investigated for the presence of BoDV-1. The shrew species were identified according to taxonomic clues and molecular barcodes. Affected horses and all shrews were investigated using histology, immunohistochemistry (IHC) and reverse transcription PCR. The horses exhibited severe nonpurulent encephalitis. Large amounts of BoDV-1 antigen were identified in their CNS. Among the 28 shrews, nine were identified as C. leucodon and 13 as Sorex araneus (Common shrew; Eurasian shrew). Six C. leucodon (66.7%) and one S. araneus (7.7%) had BoDV-1 infections. In accordance with previous findings, the IHC of C. leucodon exhibited a high amount of viral antigen in many neural and extraneural tissues. By contrast, the single positive S. araneus had an exclusively neural staining pattern. Of all positive samples, whole-genome BoDV-1 sequences were generated. The acquired sequences of the affected shrews were not identical to each other and clustered around the sequences of the diseased horses belonging, surprisingly, to the German 'strain V' cluster.
Figures



References
-
- Staeheli P, Sauder C, Hausmann J et al. Epidemiology of Borna disease virus. J Gen Virol 2000; 81: 2123–2135. - PubMed
-
- Kolodziejek J, Dürrwald R, Herzog S et al. Genetic clustering of Borna disease virus natural animal isolates, laboratory and vaccine strains strongly reflects their regional geographical origin. J Gen Virol 2005; 86: 385–398. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
