Enhancing KCC2 function counteracts morphine-induced hyperalgesia
- PMID: 28634406
- PMCID: PMC5478677
- DOI: 10.1038/s41598-017-04209-3
Enhancing KCC2 function counteracts morphine-induced hyperalgesia
Abstract
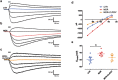
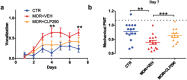
Morphine-induced hyperalgesia (MIH) is a severe adverse effect accompanying repeated morphine treatment, causing a paradoxical decrease in nociceptive threshold. Previous reports associated MIH with a decreased expression of the Cl- extruder KCC2 in the superficial dorsal horn (SDH) of the spinal cord, weakening spinal GABAA/glycine-mediated postsynaptic inhibition. Here, we tested whether the administration of small molecules enhancing KCC2, CLP257 and its pro-drug CLP290, may counteract MIH. MIH was typically expressed within 6-8 days of morphine treatment. Morphine-treated rats exhibited decreased withdrawal threshold to mechanical stimulation and increased vocalizing behavior to subcutaneous injections. Chloride extrusion was impaired in SDH neurons measured as a depolarizing shift in E GABA under Cl- load. Delivering CLP257 to spinal cord slices obtained from morphine-treated rats was sufficient to restore Cl- extrusion capacity in SDH neurons. In vivo co-treatment with morphine and oral CLP290 prevented membrane KCC2 downregulation in SDH neurons. Concurrently, co-treatment with CLP290 significantly mitigated MIH and acute administration of CLP257 in established MIH restored normal nociceptive behavior. Our data indicate that enhancing KCC2 activity is a viable therapeutic approach for counteracting MIH. Chloride extrusion enhancers may represent an effective co-adjuvant therapy to improve morphine analgesia by preventing and reversing MIH.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

