Whole Blood Profiling of Bacillus Calmette-Guérin-Induced Trained Innate Immunity in Infants Identifies Epidermal Growth Factor, IL-6, Platelet-Derived Growth Factor-AB/BB, and Natural Killer Cell Activation
- PMID: 28634479
- PMCID: PMC5459878
- DOI: 10.3389/fimmu.2017.00644
Whole Blood Profiling of Bacillus Calmette-Guérin-Induced Trained Innate Immunity in Infants Identifies Epidermal Growth Factor, IL-6, Platelet-Derived Growth Factor-AB/BB, and Natural Killer Cell Activation
Abstract
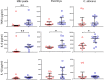
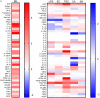
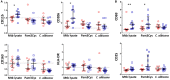
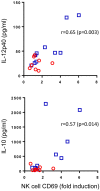
Vaccination of infants with bacillus Calmette-Guérin (BCG) activates both the innate and adaptive arms of the immune response. The antimycobacterial effects of these responses most likely account for the ability of BCG to protect against childhood forms of tuberculosis (TB). There is also evidence for a heterologous protective effect of BCG vaccination against TB-unrelated mortality in low birth weight infants. A possible mechanism of action of this effect, the induction of trained innate immunity, has been demonstrated when cells from BCG-vaccinated adults are restimulated in vitro with non-related microbial stimuli. Our aim was to examine an extensive panel of secreted immune biomarkers to characterize the profile of trained innate immunity in infants. Stimulation of whole blood for 48 h was performed 4 months after BCG vaccination, or in control unvaccinated infants. Stimulants were lipopolysaccharide; Pam3Cys (P3C); heat-killed Candida albicans, Staphylococcus aureus, Escherichia coli, and a lysate of Mycobacterium tuberculosis. Culture supernatants were tested for secreted cytokines and chemokines by 42-plex bead array and monocytes and natural killer (NK) cells assessed for expression of activation markers by flow cytometry. BCG-vaccinated infants displayed increases in 11 cytokines and chemokines in response to different non-specific innate immunity stimuli: epidermal growth factor (EGF); eotaxin; IL-6; IL-7; IL-8; IL-10; IL-12p40; monocyte chemotactic protein-3; macrophage inflammatory protein-1α; soluble CD40 ligand and platelet-derived growth factor (PDGF)-AB/BB. Although each stimulant induced a distinct response profile, three analytes, EGF, IL-6, and PDGF-AB/BB, were commonly higher after stimulation with Pam3Cys, C. albicans, and S. aureus. Conversely, certain cytokines such as interferon gamma-inducible protein-10, IL-2, IL-13, IL-17, GM-CSF, and GRO were suppressed in BCG-vaccinated infants, while no increases in TNFα or IL-1β production were detected. We did not observe a concomitant, BCG-associated change in monocyte surface activation markers in response to non-specific stimuli, but we detected a significant increase in CD69 expression on NK cells in response to Pam3Cys. Pam3Cys-induced NK cell activation correlated with the magnitude of IL-12p40 and IL-10 responses to the same stimulant. This study reveals a novel cytokine/chemokine biomarker signature of BCG-induced trained innate immunity in infants and the involvement of NK cells in these responses.
Keywords: bacillus Calmette–Guérin; chemokines; cytokines; heterologous effects; infants; natural killer; trained immunity; vaccination.
Figures

References
-
- Colditz GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, Burdick E, et al. The efficacy of bacillus Calmette-Guérin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics (1995) 96:29–35. - PubMed
-
- Black GF, Weir RE, Floyd S, Bliss L, Warndorff DK, Crampin AC, et al. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet (2002) 359:1393–401. 10.1016/S0140-6736(02)08353-8 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

