A multi-source approach to determine SMA incidence and research ready population
- PMID: 28634652
- PMCID: PMC5502065
- DOI: 10.1007/s00415-017-8549-1
A multi-source approach to determine SMA incidence and research ready population
Abstract
In spinal muscular atrophy (SMA), degeneration of motor neurons causes progressive muscular weakness, which is caused by homozygous deletion of the SMN1 gene. Available epidemiological data on SMA are scarce, often outdated, and limited to relatively small regions or populations. Combining data from different sources including genetic laboratories and patient registries may provide better insight of the disease epidemiology. To investigate the incidence of genetically confirmed SMA, and the number of patients who are able and approachable to participate in new clinical trials and observational research, we used both genetic laboratories, the TREAT-NMD Global SMA Patient Registry and the Care and Trial Sites Registry (CTSR). In Europe, 4653 patients were genetically diagnosed by the genetic laboratories in the 5-year period 2011 to 2015, with 992 diagnosed in 2015 alone. The data provide an estimated incidence of SMA in Europe of 1 in 3900-16,000 live births. Patient numbers in the national patient registries and CTSR were considerably lower. By far, most patients registered in the national patient registries and the CTSR live in Europe and are reported to have SMA type II. Considerable differences between countries in patient participation in the registries were observed. Our findings indicate that not all patients with SMA are accessed by specialist healthcare services and these patients may not have access to research opportunities and optimal care.
Keywords: Genetic laboratories; Incidence; Prevalence; Registries; Spinal muscular atrophy.
Conflict of interest statement
Conflicts of interest
Agata Robertson is the curator of the UK SMA patient registry, Rebecca Leary is the TREAT-NMD Programme and Kirsten König and Janberd Kirschner are the Care and Trial Site Registry coordinators. Cynthia C. Jones is employed by and stock holder of Biogen MA Inc. and Suzanne F. Cook is a consultant to Biogen MA Inc. The study was funded by a Grant from Biogen MA Inc.
Ethical standard
This study was approved by the institutional ethics committee. The study was conducted in accordance with the ethical standards laid down in the declaration of Helsinki of 1964 and its later amendments.
Figures
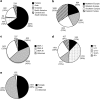
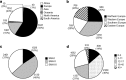
References
-
- Wirth B, Herz M, Wetter A, Moskau S, Hahnen E, Rudnik-Schoneborn S, Wienker T, Zerres K. Quantitative analysis of survival motor neuron copies: identification of subtle SMN1 mutations in patients with spinal muscular atrophy, genotype-phenotype correlation, and implications for genetic counseling. Am J Hum Genet. 1999;64(5):1340–1356. doi: 10.1086/302369. - DOI - PMC - PubMed
-
- Feldkotter M, Schwarzer V, Wirth R, Wienker TF, Wirth B. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am J Hum Genet. 2002;70(2):358–368. doi: 10.1086/338627. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

