Sorafenib in Japanese Patients with Locally Advanced or Metastatic Medullary Thyroid Carcinoma and Anaplastic Thyroid Carcinoma
- PMID: 28635560
- PMCID: PMC5646744
- DOI: 10.1089/thy.2016.0621
Sorafenib in Japanese Patients with Locally Advanced or Metastatic Medullary Thyroid Carcinoma and Anaplastic Thyroid Carcinoma
Abstract
Background: Therapeutic options for treating advanced or metastatic medullary thyroid carcinoma (MTC) and anaplastic thyroid carcinoma (ATC) are still limited in Japan, even though vandetanib for MTC and lenvatinib for MTC and ATC have been approved. Sorafenib is an oral multikinase inhibitor approved for the treatment of patients with radioactive iodine-refractory differentiated thyroid cancer (DTC). An uncontrolled, open-label, multicenter, single-arm, Phase 2 clinical study was conducted to evaluate the safety and efficacy of sorafenib in Japanese patients with MTC and ATC.
Methods: Japanese patients with histologically confirmed ATC and locally advanced or metastatic MTC were enrolled from April to September 2014. The primary endpoint was to evaluate the safety of sorafenib. Treatment efficacy variables including progression-free survival (PFS), overall survival (OS), objective response rate (ORR), disease control rate (DCR), and maximum reduction in tumor size were evaluated as secondary endpoints. Patients received sorafenib 400 mg orally twice daily on a continuous basis and then continued treatment until the occurrence of disease progression, unacceptable toxicity, or withdrawal of consent.
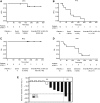
Results: A total of 20 patients were screened, and 18 (8 with MTC and 10 with ATC) were enrolled. The most common drug-related adverse events were palmar-plantar erythrodysesthesia (72%), alopecia (56%), hypertension (56%), and diarrhea (44%). In the ATC patients, median PFS was 2.8 months [confidence interval 0.7-5.6], and median OS was 5.0 months [confidence interval 0.7-5.7]; ORR and DCR were 0% and 40%, respectively. In the MTC population, neither median PFS nor OS had been reached at the time of this analysis; ORR was 25% and DCR was 75%.
Conclusions: The toxicities reported in this study were consistent with the known safety profile of sorafenib. Sorafenib seems to be effective in the treatment of advanced MTC but not ATC, and could be a new treatment option for locally advanced or metastatic MTC and radioactive iodine-refractory DTC.
Keywords: Phase II clinical trial; anaplastic thyroid carcinoma; medullary thyroid carcinoma; overall survival; progression-free survival; sorafenib.
Conflict of interest statement
K.K., I.Y., and K.T. are employees of Bayer Yakuhin Ltd. No competing financial interests exist for the remaining authors.
Figures
References
-
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Available at: https://seer.cancer.gov/statfacts/html/thyro.html (accessed February20, 2017)
-
- American Cancer Society. What is thyroid cancer? Available at: www.cancer.org/cancer/thyroidcancer/detailedguide/thyroid-cancer-what-is... (accessed November10, 2016)
-
- Saikawa M, Haruki A. 2013. Column 2. Prevalence of various histological types of thyroid cancer. In: Takami H, Ito Y, Noguchi H, Yoshida A, Okamoto T. (eds) Treatment of Thyroid Tumor: Japanese Clinical Guidelines. Spinger; Japan, p. 27
-
- Orlandi F, Caraci P, Mussa A, Saggiorato E, Pancani G, Angeli A. 2001. Treatment of medullary thyroid carcinoma: an update. Endocr Relat Cancer 8:135–147 - PubMed
-
- Union for International Cancer Control 2009. TNM Classification of Malignant Tumors. Seventh edition. Wiley-Blackwell, Hoboken, NJ
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

