The Application of Heptamethine Cyanine Dye DZ-1 and Indocyanine Green for Imaging and Targeting in Xenograft Models of Hepatocellular Carcinoma
- PMID: 28635650
- PMCID: PMC5486152
- DOI: 10.3390/ijms18061332
The Application of Heptamethine Cyanine Dye DZ-1 and Indocyanine Green for Imaging and Targeting in Xenograft Models of Hepatocellular Carcinoma
Abstract
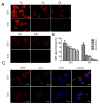
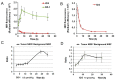
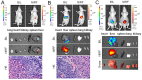
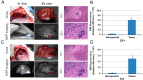
Near infrared fluorescence (NIRF) imaging has strong potential for widespread use in noninvasive tumor imaging. Indocyanine green (ICG) is the only Food and Drug Administration (FDA) -approved NIRF dye for clinical diagnosis; however, it is unstable and poorly targets tumors. DZ-1 is a novel heptamethine cyanine NIRF dye, suitable for imaging and tumor targeting. Here, we compared the fluorescence intensity and metabolism of DZ-1 and ICG. Additionally, we assayed their specificities and abilities to target tumor cells, using cultured hepatocellular carcinoma (HCC) cell lines, a nude mouse subcutaneous xenograft model of liver cancer, and a rabbit orthotopic transplantation model. We found that DZ-1 accumulates in tumor tissue and specifically recognizes HCC in subcutaneous and orthotopic models. The NIRF intensity of DZ-1 was one order of magnitude stronger than that of ICG, and DZ-1 showed excellent intraoperative tumor targeting in the rabbit model. Importantly, ICG accumulated at tumor sites, as well as in the liver and kidney. Furthermore, DZ-1 analog-gemcitabine conjugate (NIRG) exhibited similar tumor-specific targeting and imaging properties, including inhibition of tumor growth, in HCC patient-derived xenograft (PDX) mice. DZ-1 and NIRG demonstrated superior tumor-targeting specificity, compared to ICG. We show that DZ-1 is an effective molecular probe for specific imaging, targeting, and therapy in HCC.
Keywords: hepatocellular carcinoma; heptamethine cyanine dyes; intraoperative navigation; near-infrared fluorescence; optical imaging; xenograft model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Trivedi E.R., Harney A.S., Olive M.B., Podgorski I., Moin K., Sloane B.F., Barrett A.G., Meade T.J., Hoffman B.M. Chiral porphyrazine near-IR optical imaging agent exhibiting preferential tumor accumulation. Proc. Natl. Acad. Sci. USA. 2010;107:1284–1288. doi: 10.1073/pnas.0912811107. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

