Diverse Regulation of Vitamin D Receptor Gene Expression by 1,25-Dihydroxyvitamin D and ATRA in Murine and Human Blood Cells at Early Stages of Their Differentiation
- PMID: 28635660
- PMCID: PMC5486144
- DOI: 10.3390/ijms18061323
Diverse Regulation of Vitamin D Receptor Gene Expression by 1,25-Dihydroxyvitamin D and ATRA in Murine and Human Blood Cells at Early Stages of Their Differentiation
Abstract
Vitamin D receptor (VDR) is present in multiple blood cells, and the hormonal form of vitamin D, 1,25-dihydroxyvitamin D (1,25D) is essential for the proper functioning of the immune system. The role of retinoic acid receptor α (RARα) in hematopoiesis is very important, as the fusion of RARα gene with PML gene initiates acute promyelocytic leukemia where differentiation of the myeloid lineage is blocked, followed by an uncontrolled proliferation of leukemic blasts. RARα takes part in regulation of VDR transcription, and unliganded RARα acts as a transcriptional repressor to VDR gene in acute myeloid leukemia (AML) cells. This is why we decided to examine the effects of the combination of 1,25D and all-trans-retinoic acid (ATRA) on VDR gene expression in normal human and murine blood cells at various steps of their development. We tested the expression of VDR and regulation of this gene in response to 1,25D or ATRA, as well as transcriptional activities of nuclear receptors VDR and RARs in human and murine blood cells. We discovered that regulation of VDR expression in humans is different from in mice. In human blood cells at early stages of their differentiation ATRA, but not 1,25D, upregulates the expression of VDR. In contrast, in murine blood cells 1,25D, but not ATRA, upregulates the expression of VDR. VDR and RAR receptors are present and transcriptionally active in blood cells of both species, especially at early steps of blood development.
Keywords: CYP24A1; CYP26A1; blood cells; differentiation; expression; hematopoietic stem cells; retinoic acid receptors; vitamin D receptor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
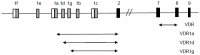


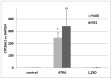
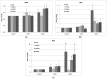
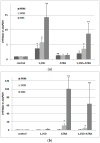




References
-
- Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

