Origin of fast ion diffusion in super-ionic conductors
- PMID: 28635958
- PMCID: PMC5482052
- DOI: 10.1038/ncomms15893
Origin of fast ion diffusion in super-ionic conductors
Abstract
Super-ionic conductor materials have great potential to enable novel technologies in energy storage and conversion. However, it is not yet understood why only a few materials can deliver exceptionally higher ionic conductivity than typical solids or how one can design fast ion conductors following simple principles. Using ab initio modelling, here we show that fast diffusion in super-ionic conductors does not occur through isolated ion hopping as is typical in solids, but instead proceeds through concerted migrations of multiple ions with low energy barriers. Furthermore, we elucidate that the low energy barriers of the concerted ionic diffusion are a result of unique mobile ion configurations and strong mobile ion interactions in super-ionic conductors. Our results provide a general framework and universal strategy to design solid materials with fast ionic diffusion.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

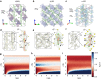
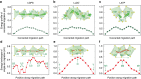

References
-
- Tarascon J.-M. & Armand M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001). - PubMed
-
- Dunn B., Kamath H. & Tarascon J.-M. Electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011). - PubMed
-
- Wachsman E. D. & Lee K. T. Lowering the temperature of solid oxide fuel cells. Science 334, 935–939 (2011). - PubMed
-
- Sunarso J. et al.. Mixed ionic–electronic conducting (MIEC) ceramic-based membranes for oxygen separation. J. Membr. Sci. 320, 13–41 (2008).
-
- Xu T. Ion exchange membranes: state of their development and perspective. J. Membr. Sci. 263, 1–29 (2005).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

