Decarboxylative Alkynylation
- PMID: 28636185
- PMCID: PMC5792189
- DOI: 10.1002/anie.201705107
Decarboxylative Alkynylation
Abstract
The development of a new decarboxylative cross-coupling method that affords terminal and substituted alkynes from various carboxylic acids is described using both nickel- and iron-based catalysts. The use of N-hydroxytetrachlorophthalimide (TCNHPI) esters is crucial to the success of the transformation, and the reaction is amenable to in situ carboxylic acid activation. Additionally, an inexpensive, commercially available alkyne source is employed in this formal homologation process that serves as a surrogate for other well-established alkyne syntheses. The reaction is operationally simple and broad in scope while providing succinct and scalable avenues to previously reported synthetic intermediates.
Keywords: alkynylation; homologation; iron catalysis; nickel catalysis; redox-active esters.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


 : RAE (1.0 equiv), NiCl2•6H2O (20 mol %), L1 (20 mol%), ethynylzinc chloride (2.5 equiv), THF/DMF, rt, 12 h. Reaction conditions with
: RAE (1.0 equiv), NiCl2•6H2O (20 mol %), L1 (20 mol%), ethynylzinc chloride (2.5 equiv), THF/DMF, rt, 12 h. Reaction conditions with
 : RAE (1.0 equiv), FeBr2•H2O (20 mol%), alkynyl Grignard (1.5 equiv), NMP/THF, 15 min, −15 °C. b) 4.0 mmol scale. c) NMR yield using internal standard. d) in situ reaction with TCNHPI (1.1 equiv) and DIC (1.1 equiv). e) 2.0 mmol scale. f) For details, see Supporting Information. g) in situ reaction with TCNHPI (1.1 equiv) and DCC (1.1 equiv).
: RAE (1.0 equiv), FeBr2•H2O (20 mol%), alkynyl Grignard (1.5 equiv), NMP/THF, 15 min, −15 °C. b) 4.0 mmol scale. c) NMR yield using internal standard. d) in situ reaction with TCNHPI (1.1 equiv) and DIC (1.1 equiv). e) 2.0 mmol scale. f) For details, see Supporting Information. g) in situ reaction with TCNHPI (1.1 equiv) and DCC (1.1 equiv).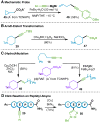

References
-
- Lam J, Breteler H, Arnason T, Hansen L, editors. Chemistry and Biology of Naturally-Occurring Acetylenes and Related Compounds. Elsevier; Amsterdam: 1988.
- Patai S, editor. Chemistry of Triple-Bonded Functional Groups. Wiley-VCH; New York: 1994.
- Stang PJ, Diederich F, editors. Modern Acetylene Chemistry. Wiley-VCH; Weinheim, Germany: 1995.
-
- Pässler P, et al. “Acetylene,” Ullman's Encyclopedia of Industrial Chemistry. Wiley-VCH; Weinheim, Germany: 2002.
- Liu Y, Lam JWY, Tang BZ. Natl Sci Rev. 2015;2:493.
- Jewett JC, Bertozzi C. Chem Soc Rev. 2010;39:1272. - PMC - PubMed
- Thirumurugan P, Matasiuk D, Jozwiak K. Chem Rev. 2013;113:4905. - PubMed
-
-
For a review, see: Habrant D, Rauhala V, Koskinen AMP. Chem Soc Rev. 2010;39:2007.
-
-
- Corey EJ, Fuchs PL. Tetrahedron Lett. 1972;13:3769.
-
- Seyferth D, Marmor RS, Hilbert P. J Org Chem. 1971;36:1379.
- Gilbert JC, Weerasooriya U. J Org Chem. 1982;47:1837.
- Ohira S. Synth Commun. 1989;19:561.
- Müller S, Liepold B, Roth G, Bestmann HJ. Synlett. 1996:521.
- Roth GJ, Liepold B, Muller SG, Bestmann HJ. Synthesis. 2004:59.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

