Selective sp3 C-H alkylation via polarity-match-based cross-coupling
- PMID: 28636596
- PMCID: PMC5655994
- DOI: 10.1038/nature22813
Selective sp3 C-H alkylation via polarity-match-based cross-coupling
Abstract
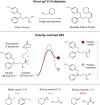
The functionalization of carbon-hydrogen (C-H) bonds is one of the most attractive strategies for molecular construction in organic chemistry. The hydrogen atom is considered to be an ideal coupling handle, owing to its relative abundance in organic molecules and its availability for functionalization at almost any stage in a synthetic sequence. Although many C-H functionalization reactions involve C(sp3)-C(sp2) coupling, there is a growing demand for C-H alkylation reactions, wherein sp3 C-H bonds are replaced with sp3 C-alkyl groups. Here we describe a polarity-match-based selective sp3 C-H alkylation via the combination of photoredox, nickel and hydrogen-atom transfer catalysis. This methodology simultaneously uses three catalytic cycles to achieve hydridic C-H bond abstraction (enabled by polarity matching), alkyl halide oxidative addition, and reductive elimination to enable alkyl-alkyl fragment coupling. The sp3 C-H alkylation is highly selective for the α-C-H of amines, ethers and sulphides, which are commonly found in pharmaceutically relevant architectures. This cross-coupling protocol should enable broad synthetic applications in de novo synthesis and late-stage functionalization chemistry.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

