Inhibition of hepatitis B viral entry by nucleic acid polymers in HepaRG cells and primary human hepatocytes
- PMID: 28636622
- PMCID: PMC5479567
- DOI: 10.1371/journal.pone.0179697
Inhibition of hepatitis B viral entry by nucleic acid polymers in HepaRG cells and primary human hepatocytes
Abstract
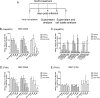
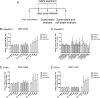
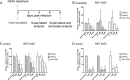
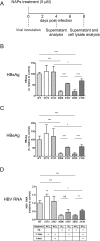
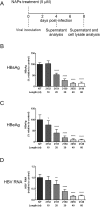
Hepatitis B virus (HBV) infection remains a major public health concern worldwide with 240 million individuals chronically infected and at risk of developing cirrhosis and hepatocellular carcinoma. Current treatments rarely cure chronic hepatitis B infection, highlighting the need for new anti-HBV drugs. Nucleic acid polymers (NAPs) are phosphorothioated oligonucleotides that have demonstrated a great potential to inhibit infection with several viruses. In chronically infected human patients, NAPs administration lead to a decline of blood HBsAg and HBV DNA and to HBsAg seroconversion, the expected signs of functional cure. NAPs have also been shown to prevent infection of duck hepatocytes with the Avihepadnavirus duck hepatitis B virus (DHBV) and to exert an antiviral activity against established DHBV infection in vitro and in vivo. In this study, we investigated the specific anti-HBV antiviral activity of NAPs in the HepaRG human hepatoma cell line and primary cultures of human hepatocytes. NAPs with different chemical features (phosphorothioation, 2'O-methyl ribose, 5-methylcytidine) were assessed for antiviral activity when provided at the time of HBV inoculation or post-inoculation. NAPs dose-dependently inhibited HBV entry in a phosphorothioation-dependent, sequence-independent and size-dependent manner. This inhibition of HBV entry by NAPs was impaired by 2'O-methyl ribose modification. NAP treatment after viral inoculation did not elicit any antiviral activity.
Conflict of interest statement
Figures
References
-
- Trépo C, Chan HLY, Lok A. Hepatitis B virus infection. Lancet Lond Engl. 2014;384: 2053–2063. doi: 10.1016/S0140-6736(14)60220-8 - DOI - PubMed
-
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet Lond Engl. 2012;380: 2095–2128. doi: 10.1016/S0140-6736(12)61728-0 - DOI - PMC - PubMed
-
- Ganem D, Prince AM. Hepatitis B virus infection—natural history and clinical consequences. N Engl J Med. 2004;350: 1118–1129. doi: 10.1056/NEJMra031087 - DOI - PubMed
-
- Kondo Y, Ninomiya M, Kakazu E, Kimura O, Shimosegawa T. Hepatitis B surface antigen could contribute to the immunopathogenesis of hepatitis B virus infection. ISRN Gastroenterol. 2013;2013: 935295 doi: 10.1155/2013/935295 - DOI - PMC - PubMed
-
- Zhu D, Liu L, Yang D, Fu S, Bian Y, Sun Z, et al. Clearing Persistent Extracellular Antigen of Hepatitis B Virus: An Immunomodulatory Strategy To Reverse Tolerance for an Effective Therapeutic Vaccination. J Immunol Baltim Md 1950. 2016;196: 3079–3087. doi: 10.4049/jimmunol.1502061 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

