Measurement properties of patient-reported outcome measures (PROMs) used in adult patients with chronic kidney disease: A systematic review
- PMID: 28636678
- PMCID: PMC5479575
- DOI: 10.1371/journal.pone.0179733
Measurement properties of patient-reported outcome measures (PROMs) used in adult patients with chronic kidney disease: A systematic review
Abstract
Background: Patient-reported outcome measures (PROMs) can provide valuable information which may assist with the care of patients with chronic kidney disease (CKD). However, given the large number of measures available, it is unclear which PROMs are suitable for use in research or clinical practice. To address this we comprehensively evaluated studies that assessed the measurement properties of PROMs in adults with CKD.
Methods: Four databases were searched; reference list and citation searching of included studies was also conducted. The COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) checklist was used to appraise the methodological quality of the included studies and to inform a best evidence synthesis for each PROM.
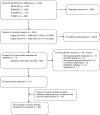
Results: The search strategy retrieved 3,702 titles/abstracts. After 288 duplicates were removed, 3,414 abstracts were screened and 71 full-text articles were retrieved for further review. Of these, 24 full-text articles were excluded as they did not meet the eligibility criteria. Following reference list and citation searching, 19 articles were retrieved bringing the total number of papers included in the final analysis to 66. There was strong evidence supporting internal consistency and moderate evidence supporting construct validity for the Kidney Disease Quality of Life-36 (KDQOL-36) in pre-dialysis patients. In the dialysis population, the KDQOL-Short Form (KDQOL-SF) had strong evidence for internal consistency and structural validity and moderate evidence for test-retest reliability and construct validity while the KDQOL-36 had moderate evidence of internal consistency, test-retest reliability and construct validity. The End Stage Renal Disease-Symptom Checklist Transplantation Module (ESRD-SCLTM) demonstrated strong evidence for internal consistency and moderate evidence for test-retest reliability, structural and construct validity in renal transplant recipients.
Conclusions: We suggest considering the KDQOL-36 for use in pre-dialysis patients; the KDQOL-SF or KDQOL-36 for dialysis patients and the ESRD-SCLTM for use in transplant recipients. However, further research is required to evaluate the measurement error, structural validity, responsiveness and patient acceptability of PROMs used in CKD.
Conflict of interest statement
References
-
- Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney international. 2011;80(12):1258–70. Epub 2011/10/14. doi: 10.1038/ki.2011.368 . - DOI - PubMed
-
- Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet (London, England). 2010;375(9731):2073–81. Epub 2010/05/21. doi: 10.1016/s0140-6736(10)60674-5 ; PubMed Central PMCID: PMCPmc3993088. - DOI - PMC - PubMed
-
- Nitsch D, Grams M, Sang Y, Black C, Cirillo M, Djurdjev O, et al. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ (Clinical research ed). 2013;346:f324 Epub 2013/01/31. doi: 10.1136/bmj.f324 ; PubMed Central PMCID: PMCPmc3558410. - DOI - PMC - PubMed
-
- Hill NR, Fatoba ST, Oke JL, Hirst JA, O'Callaghan CA, Lasserson DS, et al. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PloS one. 2016;11(7):e0158765 Epub 2016/07/08. doi: 10.1371/journal.pone.0158765 ; PubMed Central PMCID: PMCPmc4934905. - DOI - PMC - PubMed
-
- Hamer RA, El Nahas AM. The burden of chronic kidney disease: Is rising rapidly worldwide. BMJ: British Medical Journal. 2006;332(7541):563–4. PMC1397782. doi: 10.1136/bmj.332.7541.563 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical