First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer
- PMID: 28636851
- PMCID: PMC6487310
- DOI: 10.1056/NEJMoa1613493
First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer
Abstract
Background: Nivolumab has been associated with longer overall survival than docetaxel among patients with previously treated non-small-cell lung cancer (NSCLC). In an open-label phase 3 trial, we compared first-line nivolumab with chemotherapy in patients with programmed death ligand 1 (PD-L1)-positive NSCLC.
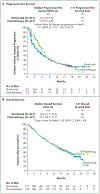
Methods: We randomly assigned, in a 1:1 ratio, patients with untreated stage IV or recurrent NSCLC and a PD-L1 tumor-expression level of 1% or more to receive nivolumab (administered intravenously at a dose of 3 mg per kilogram of body weight once every 2 weeks) or platinum-based chemotherapy (administered once every 3 weeks for up to six cycles). Patients receiving chemotherapy could cross over to receive nivolumab at the time of disease progression. The primary end point was progression-free survival, as assessed by means of blinded independent central review, among patients with a PD-L1 expression level of 5% or more.
Results: Among the 423 patients with a PD-L1 expression level of 5% or more, the median progression-free survival was 4.2 months with nivolumab versus 5.9 months with chemotherapy (hazard ratio for disease progression or death, 1.15; 95% confidence interval [CI], 0.91 to 1.45; P=0.25), and the median overall survival was 14.4 months versus 13.2 months (hazard ratio for death, 1.02; 95% CI, 0.80 to 1.30). A total of 128 of 212 patients (60%) in the chemotherapy group received nivolumab as subsequent therapy. Treatment-related adverse events of any grade occurred in 71% of the patients who received nivolumab and in 92% of those who received chemotherapy. Treatment-related adverse events of grade 3 or 4 occurred in 18% of the patients who received nivolumab and in 51% of those who received chemotherapy.
Conclusions: Nivolumab was not associated with significantly longer progression-free survival than chemotherapy among patients with previously untreated stage IV or recurrent NSCLC with a PD-L1 expression level of 5% or more. Overall survival was similar between groups. Nivolumab had a favorable safety profile, as compared with chemotherapy, with no new or unexpected safety signals. (Funded by Bristol-Myers Squibb and others; CheckMate 026 ClinicalTrials.gov number, NCT02041533 .).
Figures

Comment in
-
Lung Cancer: Frontline nivolumab - CheckMate 026 ends in stalemate.Nat Rev Clin Oncol. 2017 Jul 20;14(8):458-459. doi: 10.1038/nrclinonc.2017.102. Nat Rev Clin Oncol. 2017. PMID: 28726813 No abstract available.
-
Nivolumab as first-line treatment in non-small cell lung cancer patients-key factors: tumor mutation burden and PD-L1 ≥50.Transl Lung Cancer Res. 2018 Feb;7(Suppl 1):S28-S30. doi: 10.21037/tlcr.2018.01.04. Transl Lung Cancer Res. 2018. PMID: 29531900 Free PMC article. No abstract available.
-
Companion and complementary diagnostics for first-line immune checkpoint inhibitor treatment in non-small cell lung cancer.Transl Lung Cancer Res. 2018 Apr;7(Suppl 2):S95-S99. doi: 10.21037/tlcr.2018.02.08. Transl Lung Cancer Res. 2018. PMID: 29780701 Free PMC article. No abstract available.
Comment on
-
Cancer Immunotherapy Trials Not Immune from Imprecise Selection of Patients.N Engl J Med. 2017 Jun 22;376(25):2483-2485. doi: 10.1056/NEJMe1705692. N Engl J Med. 2017. PMID: 28636845 No abstract available.
References
-
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543–51. - PubMed
-
- Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol 2012;30: 2055–62. - PubMed
-
- Patel JD, Socinski MA, Garon EB, et al. PointBreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Clin Oncol 2013; 31:4349–57. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
