Heterogeneous Tau-Tubulin Complexes Accelerate Microtubule Polymerization
- PMID: 28636913
- PMCID: PMC5479049
- DOI: 10.1016/j.bpj.2017.05.006
Heterogeneous Tau-Tubulin Complexes Accelerate Microtubule Polymerization
Abstract
Tau is an intrinsically disordered protein with a central role in the pathology of a number of neurodegenerative diseases. Tau normally functions to stabilize neuronal microtubules, although the mechanism underlying this function is not well understood. Of note is that the interaction between tau and soluble tubulin, which has implications both in understanding tau function as well as its role in disease, is underexplored. Here we investigate the relationship between heterogeneity in tau-tubulin complexes and tau function. Specifically, we created a series of truncated and scrambled tau constructs and characterized the size and heterogeneity of the tau-tubulin complexes formed under nonpolymerizing conditions. Function of the constructs was verified by tubulin polymerization assays. We find that, surprisingly, the pseudo-repeat region of tau, which flanks the core microtubule-binding domain of tau, contributes largely to the formation of large, heterogeneous tau tubulin complexes; additional independent tubulin binding sites exist in repeats two and three of the microtubule binding domain. Of particular interest is that we find positive correlation between the size and heterogeneity of the complexes and rate of tau-promoted microtubule polymerization. We propose that tau-tubulin can be described as a "fuzzy" complex, and our results demonstrate the importance of heterogeneous complex formation in tau function. This work provides fundamental insights into the functional mechanism of tau, and more broadly underscores the relevance of heterogeneous and dynamic complexes in the functions of intrinsically disordered proteins.
Copyright © 2017 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

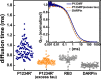

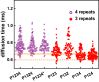

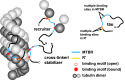
References
-
- Ballatore C., Lee V.M.Y., Trojanowski J.Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007;8:663–672. - PubMed
-
- Mazanetz M.P., Fischer P.M. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat. Rev. Drug Discov. 2007;6:464–479. - PubMed
-
- Trojanowski J.Q., Lee V.M.Y. Pathological tau: a loss of normal function or a gain in toxicity? Nat. Neurosci. 2005;8:1136–1137. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

