Transduction of Repetitive Mechanical Stimuli by Piezo1 and Piezo2 Ion Channels
- PMID: 28636944
- PMCID: PMC5646378
- DOI: 10.1016/j.celrep.2017.05.079
Transduction of Repetitive Mechanical Stimuli by Piezo1 and Piezo2 Ion Channels
Abstract
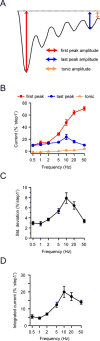
Several cell types experience repetitive mechanical stimuli, including vein endothelial cells during pulsating blood flow, inner ear hair cells upon sound exposure, and skin cells and their innervating dorsal root ganglion (DRG) neurons when sweeping across a textured surface or touching a vibrating object. While mechanosensitive Piezo ion channels have been clearly implicated in sensing static touch, their roles in transducing repetitive stimulations are less clear. Here, we perform electrophysiological recordings of heterologously expressed mouse Piezo1 and Piezo2 responding to repetitive mechanical stimulations. We find that both channels function as pronounced frequency filters whose transduction efficiencies vary with stimulus frequency, waveform, and duration. We then use numerical simulations and human disease-related point mutations to demonstrate that channel inactivation is the molecular mechanism underlying frequency filtering and further show that frequency filtering is conserved in rapidly adapting mouse DRG neurons. Our results give insight into the potential contributions of Piezos in transducing repetitive mechanical stimuli.
Keywords: Piezo1; Piezo2; dorsal root ganglia neurons; four-state gating mechanism; frequency filtering; inactivation; mechanosensitive ion channel; mechanotransduction; repetitive stimulation.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures







References
-
- Albuisson J, Murthy SE, Bandell M, Coste B, Louis-dit-Picard H, Mathur J, Feneant-Thibault M, Tertian G, de Jaureguiberry JP, Syfuss PY, et al. Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels (vol 4, pg 1884, 2013) Nature Communications 4 2013 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

