The Enteric Network: Interactions between the Immune and Nervous Systems of the Gut
- PMID: 28636959
- PMCID: PMC5551410
- DOI: 10.1016/j.immuni.2017.05.011
The Enteric Network: Interactions between the Immune and Nervous Systems of the Gut
Abstract
Interactions between the nervous and immune systems enable the gut to respond to the variety of dietary products that it absorbs, the broad spectrum of pathogens that it encounters, and the diverse microbiome that it harbors. The enteric nervous system (ENS) senses and reacts to the dynamic ecosystem of the gastrointestinal (GI) tract by translating chemical cues from the environment into neuronal impulses that propagate throughout the gut and into other organs in the body, including the central nervous system (CNS). This review will describe the current understanding of the anatomy and physiology of the GI tract by focusing on the ENS and the mucosal immune system. We highlight emerging literature that the ENS is essential for important aspects of microbe-induced immune responses in the gut. Although most basic and applied research in neuroscience has focused on the brain, the proximity of the ENS to the immune system and its interface with the external environment suggest that novel paradigms for nervous system function await discovery.
Keywords: enteric nervous system; gastrointestinal tract; gut-brain axis; intestinal microbiota; neuro-immune interactions; neuro-immunity.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
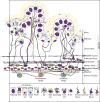



References
-
- Ahmad A, Wang CH, Bell RG. A role for IgE in intestinal immunity. Expression of rapid expulsion of Trichinella spiralis in rats transfused with IgE and thoracic duct lymphocytes. J Immunol. 1991;146:3563–3570. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

