An Essential Physiological Role for MCT8 in Bone in Male Mice
- PMID: 28637283
- PMCID: PMC5659673
- DOI: 10.1210/en.2017-00399
An Essential Physiological Role for MCT8 in Bone in Male Mice
Abstract
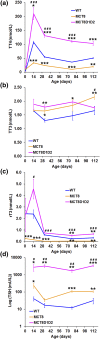
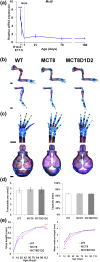
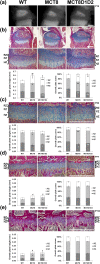
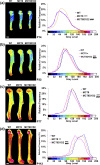
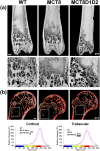
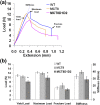
T3 is an important regulator of skeletal development and adult bone maintenance. Thyroid hormone action requires efficient transport of T4 and T3 into target cells. We hypothesized that monocarboxylate transporter (MCT) 8, encoded by Mct8 on the X-chromosome, is an essential thyroid hormone transporter in bone. To test this hypothesis, we determined the juvenile and adult skeletal phenotypes of male Mct8 knockout mice (Mct8KO) and Mct8D1D2KO compound mutants, which additionally lack the ability to convert the prohormone T4 to the active hormone T3. Prenatal skeletal development was normal in both Mct8KO and Mct8D1D2KO mice, whereas postnatal endochondral ossification and linear growth were delayed in both Mct8KO and Mct8D1D2KO mice. Furthermore, bone mass and mineralization were decreased in adult Mct8KO and Mct8D1D2KO mice, and compound mutants also had reduced bone strength. Delayed bone development and maturation in Mct8KO and Mct8D1D2KO mice is consistent with decreased thyroid hormone action in growth plate chondrocytes despite elevated serum T3 concentrations, whereas low bone mass and osteoporosis reflects increased thyroid hormone action in adult bone due to elevated systemic T3 levels. These studies identify an essential physiological requirement for MCT8 in chondrocytes, and demonstrate a role for additional transporters in other skeletal cells during adult bone maintenance.
Figures


References
-
- Bernal J, Guadaño-Ferraz A, Morte B. Thyroid hormone transporters--functions and clinical implications. Nat Rev Endocrinol. 2015;11(7):406–417. - PubMed
-
- Waung JA, Bassett JHD, Williams GR. Thyroid hormone metabolism in skeletal development and adult bone maintenance. Trends Endocrinol Metab. 2012;23(4):155–162. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

