The emergence of mesencephalic trigeminal neurons
- PMID: 28637511
- PMCID: PMC5480199
- DOI: 10.1186/s13064-017-0088-z
The emergence of mesencephalic trigeminal neurons
Abstract
Background: The cells of the mesencephalic trigeminal nucleus (MTN) are the proprioceptive sensory neurons that innervate the jaw closing muscles. These cells differentiate close to the two key signalling centres that influence the dorsal midbrain, the isthmus, which mediates its effects via FGF and WNT signalling and the roof plate, which is a major source of BMP signalling as well as WNT signalling.
Methods: In this study, we have set out to analyse the importance of FGF, WNT and BMP signalling for the development of the MTN. We have employed pharmacological inhibitors of these pathways in explant cultures as well as utilising the electroporation of inhibitory constructs in vivo in the chick embryo.
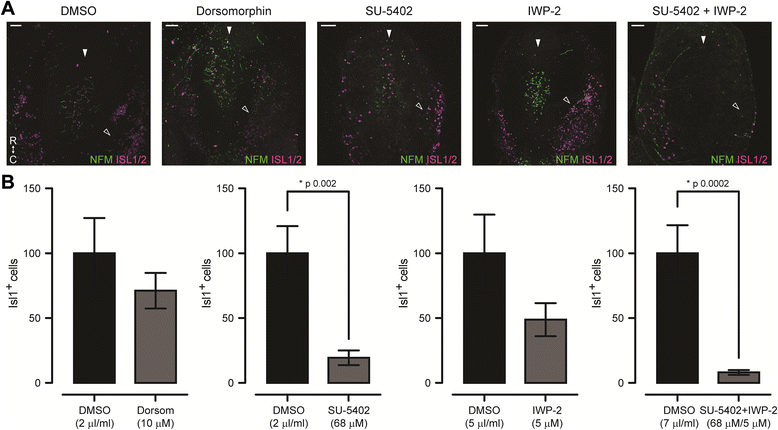
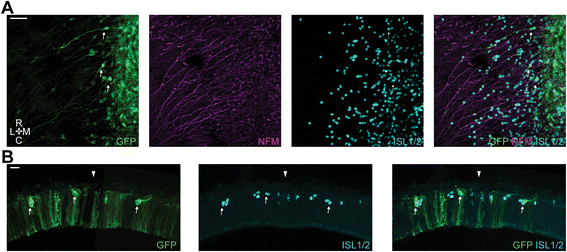
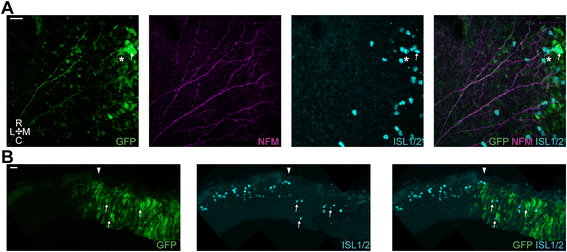
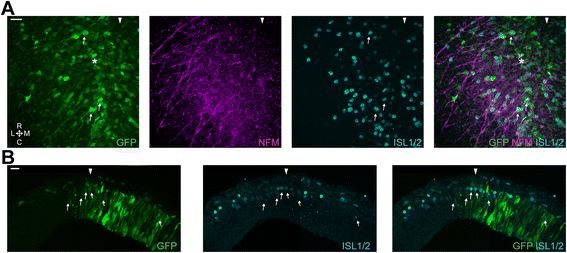
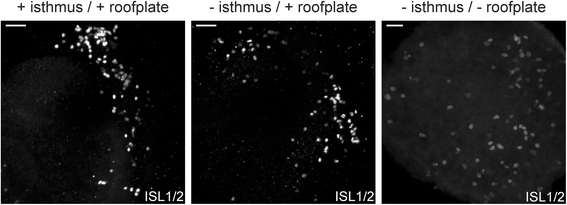
Results: We find that interfering with either FGF or WNT signalling has pronounced effects on MTN development whilst abrogation of BMP signalling has no effect. We show that treatment of explants with either FGF or WNT antagonists results in the generation of fewer MTN neurons and affects MTN axon extension and that inhibition of both these pathways has an additive effect. To complement these studies, we have used in vivo electroporation to inhibit BMP, FGF and WNT signalling within dorsal midbrain cells prior to, and during, their differentiation as MTN neurons. Again, we find that inhibition of BMP signalling has no effect on the development of MTN neurons. We additionally find that cells electroporated with inhibitory constructs for either FGF or WNT signalling can differentiate as MTN neurons suggesting that these pathways are not required cell intrinsically for the emergence of these neurons. Indeed, we also show that explants of dorsal mesencephalon lacking both the isthmus and roof plate can generate MTN neurons. However, we did find that inhibiting FGF or WNT signalling had consequences for MTN differentiation.
Conclusions: Our results suggest that the emergence of MTN neurons is an intrinsic property of the dorsal mesencephalon of gnathostomes, and that this population undergoes expansion, and maturation, along with the rest of the dorsal midbrain under the influence of FGF and WNT signalling.
Keywords: FGF; Jaw proprioception; MTN; MesV; Mesencephalic trigeminal nucleus; Midbrain; WNT BMP.
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

