A randomised, double-blind trial to demonstrate bioequivalence of GP2013 and reference rituximab combined with methotrexate in patients with active rheumatoid arthritis
- PMID: 28637670
- PMCID: PMC5561377
- DOI: 10.1136/annrheumdis-2017-211281
A randomised, double-blind trial to demonstrate bioequivalence of GP2013 and reference rituximab combined with methotrexate in patients with active rheumatoid arthritis
Abstract
Objectives: The aim of this report is to demonstrate pharmacokinetic (PK) and pharmacodynamic (PD) equivalence as well as similar efficacy, safety and immunogenicity between GP2013, a biosimilar rituximab, and innovator rituximab (RTX) in patients with rheumatoid arthritis (RA) with inadequate response or intolerance to tumour necrosis factor inhibitor (TNFi) treatment.
Methods: In this multinational, randomised, double-blind, parallel-group study, 312 patients with active disease despite prior TNFi therapy were randomised to receive GP2013 or either the EU (RTX-EU) or the US (RTX-US) reference product, along with methotrexate (MTX) and folic acid. The primary endpoint was the area under the serum concentration-time curve from study drug infusion to infinity (AUC0-inf). Additional PK and PD parameters, along with efficacy, immunogenicity and safety outcomes were also assessed up to week 24.
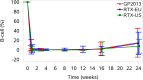
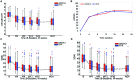
Results: The 90% CI of the geometric mean ratio of the AUCs were within the bioequivalence limits of 80% to 125% for all three comparisons; GP2013 versus RTX-EU: 1.106 (90% CI 1.010 to 1.210); GP2013 versus RTX-US: 1.012 (90% CI 0.925 to 1.108); and RTX-EU versus RTX-US: 1.093 (90% CI 0.989 to 1.208). Three-way PD equivalence of B cell depletion was also demonstrated. Efficacy, safety and immunogenicity profiles were similar between GP2013 and RTX.
Conclusions: Three-way PK/PD equivalence of GP2013, RTX-EU and RTX-US was demonstrated. Efficacy, safety and immunogenicity profiles were similar between GP2013 and RTX.
Trial registration number: NCT01274182; Results.
Keywords: B cells; DMARDs (biologic); Rheumatoid arthritis.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: PZ, LC and TS are employees of Sandoz/Hexal. JSS, HPT, AK, AB, JG-R and MS received investigator fees from Sandoz, a Novartis Division.
Figures
Similar articles
-
Pharmacokinetic Similarity and Comparative Pharmacodynamics, Safety, Efficacy, and Immunogenicity of DRL_RI Versus Reference Rituximab in Biologics-Naïve Patients with Moderate-to-Severe Rheumatoid Arthritis: A Double-Blind, Randomized, Three-Arm Study.BioDrugs. 2020 Apr;34(2):183-196. doi: 10.1007/s40259-020-00406-1. BioDrugs. 2020. PMID: 32052313 Free PMC article. Clinical Trial.
-
Comparison of biosimilar CT-P10 and innovator rituximab in patients with rheumatoid arthritis: a randomized controlled Phase 3 trial.MAbs. 2018 Aug/Sep;10(6):934-943. doi: 10.1080/19420862.2018.1487912. Epub 2018 Jul 16. MAbs. 2018. PMID: 30010481 Free PMC article. Clinical Trial.
-
Brief Report: Safety and Immunogenicity of Rituximab Biosimilar GP 2013 After Switch From Reference Rituximab in Patients With Active Rheumatoid Arthritis.Arthritis Care Res (Hoboken). 2019 Jan;71(1):88-94. doi: 10.1002/acr.23771. Arthritis Care Res (Hoboken). 2019. PMID: 30295429 Clinical Trial.
-
GP2013: A Rituximab Biosimilar.BioDrugs. 2017 Oct;31(5):465-468. doi: 10.1007/s40259-017-0245-2. BioDrugs. 2017. PMID: 28921160 Review.
-
Scientific rationale underpinning the development of biosimilar rituximab in hematological cancers and inflammatory diseases.Future Oncol. 2019 Dec;15(36):4223-4234. doi: 10.2217/fon-2019-0430. Epub 2019 Nov 13. Future Oncol. 2019. PMID: 31718287 Review.
Cited by
-
A Developer's Perspective on Clinical Evidence and Benefits for Rituximab Biosimilar Uptake, with a Focus on CT-P10.Clin Drug Investig. 2022 Apr;42(4):285-300. doi: 10.1007/s40261-022-01133-x. Epub 2022 Mar 24. Clin Drug Investig. 2022. PMID: 35325438 Review.
-
Trial Design and Statistical Considerations on the Assessment of Pharmacodynamic Similarity.AAPS J. 2019 Apr 3;21(3):47. doi: 10.1208/s12248-019-0321-2. AAPS J. 2019. PMID: 30945035
-
Pathogenesis of rheumatoid arthritis and its treatment with anti-inflammatory natural products.Mol Biol Rep. 2023 May;50(5):4687-4706. doi: 10.1007/s11033-023-08406-4. Epub 2023 Apr 6. Mol Biol Rep. 2023. PMID: 37022525 Review.
-
Real-world experience of rituximab biosimilar GP2013 in rheumatoid arthritis patients naïve to or switched from reference rituximab.Rheumatol Int. 2023 May;43(5):881-888. doi: 10.1007/s00296-023-05307-4. Epub 2023 Mar 16. Rheumatol Int. 2023. PMID: 36922417 Free PMC article.
-
Turkish League Against Rheumatism (TLAR) Recommendations for the Pharmacological Management of Rheumatoid Arthritis: 2018 Update Under Guidance of Current Recommendations.Arch Rheumatol. 2018 Jul 9;33(3):251-271. doi: 10.5606/ArchRheumatol.2018.6911. eCollection 2018 Sep. Arch Rheumatol. 2018. PMID: 30632540 Free PMC article.
References
-
- Rituxan® (rituximab) full Prescribing Information, Genentech, Inc. 2014. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103705s5311lbl.pdf
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

