Paradoxical Suppression of Atherosclerosis in the Absence of microRNA-146a
- PMID: 28637783
- PMCID: PMC5542783
- DOI: 10.1161/CIRCRESAHA.116.310529
Paradoxical Suppression of Atherosclerosis in the Absence of microRNA-146a
Abstract
Rationale: Inflammation is a key contributor to atherosclerosis. MicroRNA-146a (miR-146a) has been identified as a critical brake on proinflammatory nuclear factor κ light chain enhancer of activated B cells signaling in several cell types, including endothelial cells and bone marrow (BM)-derived cells. Importantly, miR-146a expression is elevated in human atherosclerotic plaques, and polymorphisms in the miR-146a precursor have been associated with risk of coronary artery disease.
Objective: To define the role of endogenous miR-146a during atherogenesis.
Methods and results: Paradoxically, Ldlr-/- (low-density lipoprotein receptor null) mice deficient in miR-146a develop less atherosclerosis, despite having highly elevated levels of circulating proinflammatory cytokines. In contrast, cytokine levels are normalized in Ldlr-/-;miR-146a-/- mice receiving wild-type BM transplantation, and these mice have enhanced endothelial cell activation and elevated atherosclerotic plaque burden compared with Ldlr-/- mice receiving wild-type BM, demonstrating the atheroprotective role of miR-146a in the endothelium. We find that deficiency of miR-146a in BM-derived cells precipitates defects in hematopoietic stem cell function, contributing to extramedullary hematopoiesis, splenomegaly, BM failure, and decreased levels of circulating proatherogenic cells in mice fed an atherogenic diet. These hematopoietic phenotypes seem to be driven by unrestrained inflammatory signaling that leads to the expansion and eventual exhaustion of hematopoietic cells, and this occurs in the face of lower levels of circulating low-density lipoprotein cholesterol in mice lacking miR-146a in BM-derived cells. Furthermore, we identify sortilin-1(Sort1), a known regulator of circulating low-density lipoprotein levels in humans, as a novel target of miR-146a.
Conclusions: Our study reveals that miR-146a regulates cholesterol metabolism and tempers chronic inflammatory responses to atherogenic diet by restraining proinflammatory signaling in endothelial cells and BM-derived cells.
Keywords: atherosclerosis; endothelial cells; hematopoiesis; inflammation; microRNAs.
© 2017 The Authors.
Figures

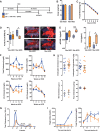

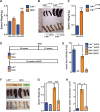




Comment in
-
MicroRNA-146a & hematopoiesis: friend or foe in atherosclerosis.Noncoding RNA Investig. 2018 Jul;2:43. doi: 10.21037/ncri.2018.06.08. Epub 2018 Jul 17. Noncoding RNA Investig. 2018. PMID: 30101215 Free PMC article. No abstract available.
References
-
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. - PubMed
-
- Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. - PubMed
-
- Gareus R, Kotsaki E, Xanthoulea S, van der Made I, Gijbels MJ, Kardakaris R, Polykratis A, Kollias G, de Winther MP, Pasparakis M. Endothelial cell-specific NF-kappaB inhibition protects mice from atherosclerosis. Cell Metab. 2008;8:372–383. doi: 10.1016/j.cmet.2008.08.016. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

