Store-operated calcium entry suppressed the TGF-β1/Smad3 signaling pathway in glomerular mesangial cells
- PMID: 28637791
- PMCID: PMC5625109
- DOI: 10.1152/ajprenal.00483.2016
Store-operated calcium entry suppressed the TGF-β1/Smad3 signaling pathway in glomerular mesangial cells
Abstract
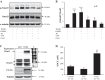
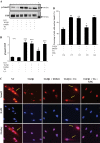
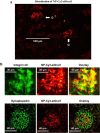
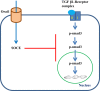
Our previous study demonstrated that the abundance of extracellular matrix proteins was suppressed by store-operated Ca2+ entry (SOCE) in mesangial cells (MCs). The present study was conducted to investigate the underlying mechanism focused on the transforming growth factor-β1 (TGF-β1)/Smad3 pathway, a critical pathway for ECM expansion in diabetic kidneys. We hypothesized that SOCE suppressed ECM protein expression by inhibiting this pathway in MCs. In cultured human MCs, we observed that TGF-β1 (5 ng/ml for 15 h) significantly increased Smad3 phosphorylation, as evaluated by immunoblot. However, this response was markedly inhibited by thapsigargin (1 µM), a classical activator of store-operated Ca2+ channels. Consistently, both immunocytochemistry and immunoblot showed that TGF-β1 significantly increased nuclear translocation of Smad3, which was prevented by pretreatment with thapsigargin. Importantly, the thapsigargin effect was reversed by lanthanum (La3+; 5 µM) and GSK-7975A (10 µM), both of which are selective blockers of store-operated Ca2+ channels. Furthermore, knockdown of Orai1, the pore-forming subunit of the store-operated Ca2+ channels, significantly augmented TGF-β1-induced Smad3 phosphorylation. Overexpression of Orai1 augmented the inhibitory effect of thapsigargin on TGF-β1-induced phosphorylation of Smad3. In agreement with the data from cultured MCs, in vivo knockdown of Orai1 specific to MCs using a targeted nanoparticle small interfering RNA delivery system resulted in a marked increase in abundance of phosphorylated Smad3 and in nuclear translocation of Smad3 in the glomerulus of mice. Taken together, our results indicate that SOCE in MCs negatively regulates the TGF-β1/Smad3 signaling pathway.
Keywords: Orai1; Smad3; mesangial cells; store-operated Ca2+ entry; transforming growth factor-β1.
Copyright © 2017 the American Physiological Society.
Figures




References
-
- Abdel-Wahab N, Wicks SJ, Mason RM, Chantry A. Decorin suppresses transforming growth factor-β-induced expression of plasminogen activator inhibitor-1 in human mesangial cells through a mechanism that involves Ca2+-dependent phosphorylation of Smad2 at serine-240. Biochem J 362: 643–649, 2002. doi: 10.1042/bj3620643. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

