Bioactive Immune Components of Anti-Diarrheagenic Enterotoxigenic Escherichia coli Hyperimmune Bovine Colostrum Products
- PMID: 28637804
- PMCID: PMC5583472
- DOI: 10.1128/CVI.00186-16
Bioactive Immune Components of Anti-Diarrheagenic Enterotoxigenic Escherichia coli Hyperimmune Bovine Colostrum Products
Abstract
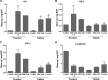
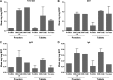
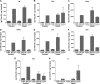
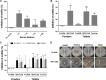
Diarrhea is a common illness among travelers to resource-limited countries, the most prevalent attributable agent being enterotoxigenic Escherichia coli (ETEC). At this time, there are no vaccines licensed specifically for the prevention of ETEC-induced traveler's diarrhea (TD), and this has propelled investigation of alternative preventive methods. Colostrum, the first milk expressed after birthing, is rich in immunoglobulins and innate immune components for protection of newborns against infectious agents. Hyperimmune bovine colostrum (HBC) produced by immunization of cows during gestation (and containing high levels of specific antibodies) is a practical and effective prophylactic tool against gastrointestinal illnesses. A commercial HBC product, Travelan, is available for prevention of ETEC-induced diarrhea. Despite its demonstrated clinical efficacy, the underlying immune components and antimicrobial activity that contribute to protection remain undefined. We investigated innate and adaptive immune components of several commercial HBC products formulated to reduce the risk of ETEC-induced diarrhea, including Travelan and IMM-124E, a newer product that has broader gastrointestinal health benefits. The immune components measured included total and ETEC-specific IgG, total IgA, cytokines, growth factors, and lactoferrin. HBC products contained high levels of IgG specific for multiple ETEC antigens, including O-polysaccharide 78 and colonization factor antigen I (CFA/I) present in the administered vaccines. Antimicrobial activity was measured in vitro using novel functional assays. HBC greatly reduced ETEC motility in soft agar and exhibited bactericidal activity in the presence of complement. We have identified immune components and antimicrobial activity potentially involved in the prevention of ETEC infection by HBC in vivo.
Keywords: bactericidal activity; enterotoxigenic E. coli; functional antibodies; hyperimmune colostrum.
Copyright © 2017 Sears et al.
Figures
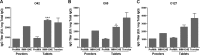

References
-
- Shah N, DuPont HL, Ramsey DJ. 2009. Global etiology of travelers' diarrhea: systematic review from 1973 to the present. Am J Trop Med Hyg 80:609–614. - PubMed
-
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

