Direct demonstration of a neonatal Fc receptor (FcRn)-driven endosomal sorting pathway for cellular recycling of albumin
- PMID: 28637874
- PMCID: PMC5555191
- DOI: 10.1074/jbc.M117.794248
Direct demonstration of a neonatal Fc receptor (FcRn)-driven endosomal sorting pathway for cellular recycling of albumin
Abstract
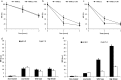
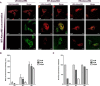
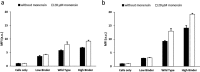
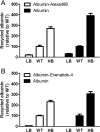
Albumin is the most abundant plasma protein involved in the transport of many compounds, such as fatty acids, bilirubin, and heme. The endothelial cellular neonatal Fc receptor (FcRn) has been suggested to play a central role in maintaining high albumin plasma levels through a cellular recycling pathway. However, direct mapping of this process is still lacking. This work presents the use of wild-type and engineered recombinant albumins with either decreased or increased FcRn affinity in combination with a low or high FcRn-expressing endothelium cell line to clearly define the FcRn involvement, intracellular pathway, and kinetics of albumin trafficking by flow cytometry, quantitative confocal microscopy, and an albumin-recycling assay. We found that cellular albumin internalization was proportional to FcRn expression and albumin-binding affinity. Albumin accumulation in early endosomes was independent of FcRn-binding affinity, but differences in FcRn-binding affinities significantly affected the albumin distribution between late endosomes and lysosomes. Unlike albumin with low FcRn-binding affinity, albumin with high FcRn-binding affinity was directed less to the lysosomes, suggestive of FcRn-directed albumin salvage from lysosomal degradation. Furthermore, the amount of recycled albumin in cell culture media corresponded to FcRn-binding affinity, with a ∼3.3-fold increase after 1 h for the high FcRn-binding albumin variant compared with wild-type albumin. Together, these findings uncover an FcRn-dependent endosomal cellular-sorting pathway that has great importance in describing fundamental mechanisms of intracellular albumin recycling and the possibility to tune albumin-based therapeutic effects by FcRn-binding affinity.
Keywords: Fc receptor; albumin; cellular recycling; endosome; intracellular processing; intracellular trafficking; receptor recycling.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Peters T. (1996) All about Albumin: Biochemistry, Genetics, and Medical Applications. Academic Press, San Diego, CA
-
- Roopenian D. C., Christianson G. J., Sproule T. J., Brown A. C., Akilesh S., Jung N., Petkova S., Avanessian L., Choi E. Y., Shaffer D. J., Eden P. A., and Anderson C. L. (2003) The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J. Immunol. 170, 3528–3533 - PubMed
-
- Ward E. S., Zhou J., Ghetie V., and Ober R. J. (2003) Evidence to support the cellular mechanism involved in serum IgG homeostasis in humans. Int. Immunol. 15, 187–195 - PubMed
-
- Chaudhury C., Brooks C. L., Carter D. C., Robinson J. M., and Anderson C. L. (2006) Albumin binding to FcRn: distinct from the FcRn-IgG interaction. Biochemistry 45, 4983–4990 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

