Global profiling of phytohormone dynamics during combined drought and pathogen stress in Arabidopsis thaliana reveals ABA and JA as major regulators
- PMID: 28638069
- PMCID: PMC5479852
- DOI: 10.1038/s41598-017-03907-2
Global profiling of phytohormone dynamics during combined drought and pathogen stress in Arabidopsis thaliana reveals ABA and JA as major regulators
Abstract
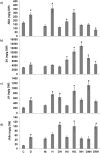
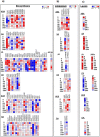
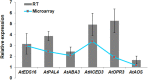
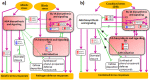
Global transcriptome studies demonstrated the existence of unique plant responses under combined stress which are otherwise not seen during individual stresses. In order to combat combined stress plants use signaling pathways and 'cross talk' mediated by hormones involved in stress and growth related processes. However, interactions among hormones' pathways in combined stressed plants are not yet known. Here we studied dynamics of different hormones under individual and combined drought and pathogen infection in Arabidopsis thaliana by liquid chromatography-mass spectrometry (LC-MS) based profiling. Our results revealed abscisic acid (ABA) and salicylic acid (SA) as key regulators under individual drought and pathogen stress respectively. Under combined drought and host pathogen stress (DH) we observed non-induced levels of ABA with an upsurge in SA and jasmonic acid (JA) concentrations, underscoring their role in basal tolerance against host pathogen. Under a non-host pathogen interaction with drought (DNH) stressed plants, ABA, SA and JA profiles were similar to those under DH or non-host pathogen alone. We propose that plants use SA/JA dependent signaling during DH stress which antagonize ABA biosynthesis and signaling pathways during early stage of stress. The study provides insights into hormone modulation at different time points during combined stress.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Ramegowda V, Senthil-Kumar M. The interactive effects of simultaneous biotic and abiotic stresses on plants: Mechanistic understanding from drought and pathogen combination. J. Plant Physiol. 2014;176C:47–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

