The application of ultrasound in detecting lymph nodal recurrence in the treated neck of head and neck cancer patients
- PMID: 28638103
- PMCID: PMC5479791
- DOI: 10.1038/s41598-017-04039-3
The application of ultrasound in detecting lymph nodal recurrence in the treated neck of head and neck cancer patients
Abstract
Early detection of neck lymph node (LN) recurrence is paramount in improving the prognosis of treated head and neck cancer patients. Ultrasound (US) with US-guided fine needle aspiration (FNA) and core needle biopsy (CNB) have been shown to have great accuracy for LN diagnoses in the untreated neck. However, in the treated neck with fibrosis, their roles are not clarified. Here, we retrospectively review 153 treated head and neck cancer patients who had received US and US-guided FNA/CNB. In multivariate logistic regression analyses, size (short-axis diameter >0.8 cm) (odds ratio (OR) 4.19, P = 0.007), round shape (short/long axis ratio >0.5) (OR 3.44, P = 0.03), heterogeneous internal echo (OR 3.92, P = 0.009) and irregular margin (OR 7.32, P < 0.001) are effective US features in predicting recurrent LNs in the treated neck. However, hypoechogenicity (OR 2.38, P = 0.289) and chaotic/absent vascular pattern (OR 3.04, P = 0.33) are ineffective. US-guided FNA (sensitivity/specificity: 95.24%/97.92%) is effective in the treated neck, though with high non-diagnostic rate (29.69%). US-guided CNB (sensitivity/specificity: 84.62%/100%) is also effective, though with low negative predictive value (62.5%). Overall, US with US-guided FNA/CNB are still effective diagnostic tools for neck nodal recurrence surveillance.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
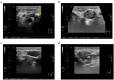
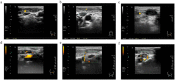

Similar articles
-
The Applications and Potential Developments of Ultrasound in Oral Cancer Management.Technol Cancer Res Treat. 2022 Jan-Dec;21:15330338221133216. doi: 10.1177/15330338221133216. Technol Cancer Res Treat. 2022. PMID: 36254559 Free PMC article. Review.
-
Impact of sentinel lymph node biopsy by ultrasound-guided core needle biopsy for patients with suspicious node positive breast cancer.Breast Cancer. 2018 Jan;25(1):86-93. doi: 10.1007/s12282-017-0795-7. Epub 2017 Jul 22. Breast Cancer. 2018. PMID: 28735457
-
US-guided core-needle biopsy versus US-guided fine-needle aspiration of suspicious cervical lymph nodes for staging workup of non-head and neck malignancies: A propensity score matching study.J Surg Oncol. 2017 Dec;116(7):870-876. doi: 10.1002/jso.24747. Epub 2017 Jun 26. J Surg Oncol. 2017. PMID: 28650524
-
Accuracy of Ultrasonography-Guided Fine-Needle Aspiration in Detecting Persistent Nodal Disease After Chemoradiotherapy.JAMA Otolaryngol Head Neck Surg. 2016 Apr;142(4):377-82. doi: 10.1001/jamaoto.2015.3934. JAMA Otolaryngol Head Neck Surg. 2016. PMID: 26967355
-
Neck Procedures: Thyroid and Parathyroid.Radiol Clin North Am. 2020 Nov;58(6):1085-1098. doi: 10.1016/j.rcl.2020.07.005. Epub 2020 Sep 17. Radiol Clin North Am. 2020. PMID: 33040850 Review.
Cited by
-
The Applications and Potential Developments of Ultrasound in Oral Cancer Management.Technol Cancer Res Treat. 2022 Jan-Dec;21:15330338221133216. doi: 10.1177/15330338221133216. Technol Cancer Res Treat. 2022. PMID: 36254559 Free PMC article. Review.
-
Quantitative ultrasound radiomics in predicting recurrence for patients with node-positive head-neck squamous cell carcinoma treated with radical radiotherapy.Cancer Med. 2021 Apr;10(8):2579-2589. doi: 10.1002/cam4.3634. Epub 2020 Dec 13. Cancer Med. 2021. PMID: 33314716 Free PMC article.
-
Assessment of clinical radiosensitivity in patients with head-neck squamous cell carcinoma from pre-treatment quantitative ultrasound radiomics.Sci Rep. 2021 Mar 17;11(1):6117. doi: 10.1038/s41598-021-85221-6. Sci Rep. 2021. PMID: 33731738 Free PMC article. Clinical Trial.
-
Risk Factors Analysis of Pathologically Confirmed Cervical Lymph Nodes Metastasis in Oral Squamous Cell Carcinoma Patients with Clinically Negative Cervical Lymph Node: Results from a Cancer Center of Central China.J Cancer. 2019 Jun 2;10(13):3062-3069. doi: 10.7150/jca.30502. eCollection 2019. J Cancer. 2019. PMID: 31281484 Free PMC article.
-
Ultrasound and nanomaterial: an efficient pair to fight cancer.J Nanobiotechnology. 2022 Mar 18;20(1):139. doi: 10.1186/s12951-022-01243-w. J Nanobiotechnology. 2022. PMID: 35300712 Free PMC article. Review.
References
-
- Liao LJ, Wang CT, Young YH, Cheng PW. Real-time and computerized sonographic scoring system for predicting malignant cervical lymphadenopathy. Head & Neck. 2010;32:594–598. - PubMed
-
- Gupta A, et al. Sonographic assessment of cervical lymphadenopathy: role of high-resolution and color Doppler imaging. Head & Neck. 2011;33:297–302. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

