A novel and effective method to generate human porcine-specific regulatory T cells with high expression of IL-10, TGF-β1 and IL-35
- PMID: 28638110
- PMCID: PMC5479824
- DOI: 10.1038/s41598-017-04322-3
A novel and effective method to generate human porcine-specific regulatory T cells with high expression of IL-10, TGF-β1 and IL-35
Abstract
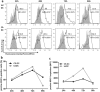
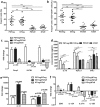
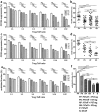
Organ transplantation remains the most effective treatment for patients with late stage organ failure. Transgenic pigs provide an alternative organ donor source to the limited availability of human organs. However, cellular rejection still remains to be the obstacle for xenotransplantation. Superior to other methods, antigen-specific regulatory T cells (Treg) alleviate cellular rejection with fewer side effects. Here we demonstrate the use of a fast method to provide tolerogenic dendritic cells (tolDC) that can be used to generate effective porcine-specific Treg cells (PSTreg). TolDC were produced within three days from human monocytes in medium supplemented with anti-inflammatory cytokines. Treg were generated from naïve CD4+ T cells and induced to become PSTreg by cocultivation with porcine-antigen-loaded tolDC. Results showed that PSTreg exhibited the expected phenotype, CD4+CD25+CD127low/- Foxp3+, and a more activated phenotype. The specificity of PSTreg was demonstrated by suppression of effector T cell (Teff) activation markers of different stages and inhibition of Teff cell proliferation. TolDC and PSTreg exhibited high expression of IL-10 and TGF-β1 at both protein and RNA levels, and PSTreg also highly expressed IL-35 at RNA levels. Upon restimulation, PSTreg retained the activated phenotype and specificity. Taken together, the newly developed procedure allows efficient generation of highly suppressive PSTreg.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Induction of porcine-specific regulatory T cells with high specificity and expression of IL-10 and TGF-β1 using baboon-derived tolerogenic dendritic cells.Xenotransplantation. 2018 Jan;25(1). doi: 10.1111/xen.12355. Epub 2017 Oct 22. Xenotransplantation. 2018. PMID: 29057512
-
TGF-β1 and contact mediated suppression by CD4+CD25+CD127- T regulatory cells of patients with self-limiting hepatitis E.Hum Immunol. 2016 Dec;77(12):1254-1263. doi: 10.1016/j.humimm.2016.10.001. Epub 2016 Oct 6. Hum Immunol. 2016. PMID: 27720959
-
Tolerogenic dendritic cells generated with dexamethasone and vitamin D3 regulate rheumatoid arthritis CD4+ T cells partly via transforming growth factor-β1.Clin Exp Immunol. 2017 Jan;187(1):113-123. doi: 10.1111/cei.12870. Epub 2016 Nov 2. Clin Exp Immunol. 2017. PMID: 27667787 Free PMC article.
-
CD4+CD25+ T Regulatory Cells in Transplantation Tolerance: 25 Years On.Transplantation. 2016 Dec;100(12):2533-2547. doi: 10.1097/TP.0000000000001436. Transplantation. 2016. PMID: 27861285 Review.
-
Distinct regulatory CD4+T cell subsets; differences between naïve and antigen specific T regulatory cells.Curr Opin Immunol. 2011 Oct;23(5):641-7. doi: 10.1016/j.coi.2011.07.012. Epub 2011 Aug 11. Curr Opin Immunol. 2011. PMID: 21840184 Review.
Cited by
-
Regulatory cell therapy for kidney transplantation and autoimmune kidney diseases.Pediatr Nephrol. 2025 Jan;40(1):39-52. doi: 10.1007/s00467-024-06514-2. Epub 2024 Sep 16. Pediatr Nephrol. 2025. PMID: 39278988 Free PMC article. Review.
-
The prognostic value of intratumoral and peritumoral tumor-infiltrating FoxP3+Treg cells in of pancreatic adenocarcinoma: a meta-analysis.World J Surg Oncol. 2021 Oct 16;19(1):300. doi: 10.1186/s12957-021-02420-1. World J Surg Oncol. 2021. PMID: 34654443 Free PMC article.
-
Resistance Mechanism of PD-1/PD-L1 Blockade in the Cancer-Immunity Cycle.Onco Targets Ther. 2020 Jan 7;13:83-94. doi: 10.2147/OTT.S239398. eCollection 2020. Onco Targets Ther. 2020. PMID: 32021257 Free PMC article. Review.
-
TRIM41 contributes to the pathogenesis of airway allergy by compromising dendritic cells' tolerogenic properties.iScience. 2024 May 21;27(6):110067. doi: 10.1016/j.isci.2024.110067. eCollection 2024 Jun 21. iScience. 2024. PMID: 38883815 Free PMC article.
-
Overcoming acquired resistance to cancer immune checkpoint therapy: potential strategies based on molecular mechanisms.Cell Biosci. 2023 Jun 30;13(1):120. doi: 10.1186/s13578-023-01073-9. Cell Biosci. 2023. PMID: 37386520 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

