The structure-function relationship of disulfide bonds in etanercept
- PMID: 28638112
- PMCID: PMC5479810
- DOI: 10.1038/s41598-017-04320-5
The structure-function relationship of disulfide bonds in etanercept
Abstract
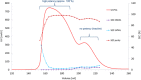
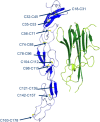
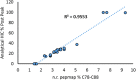
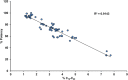
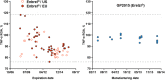
Etanercept is a TNFα receptor Fc fusion protein used for the treatment of rheumatic disease and psoriasis. Physicochemical and functional investigation of process fractions during development of the etanercept biosimilar GP2015 (Erelzi®) revealed a correlation between reduced potency and incorrect disulfide bridging between specific cysteines in the receptor domain. This novel structure-function relationship was found to be the molecular basis for reduced potency in recent Enbrel® batches, which exhibit higher levels of incorrect disulfide bridging. Interestingly, incorrect disulfide bridging was found to be reversible under serum-like redox conditions, restoring potency to normal levels. This redox dependent reversibility suggests that these variants are likely not relevant for clinical efficacy once the drug enters the bloodstream. Nonetheless, incorrect disulfide bridging in etanercept represents a new quality attribute that is critical for biopharmaceutical functionality and should thus be carefully monitored and controlled to guarantee patient safety.
Conflict of interest statement
Erelzi® is a registered trademark of Novartis AG and is, at the time of this submission, approved exclusively in the USA. The Erelzi trademark is registered worldwide. Enbrel® is a registered trademark of Immunex Corporation and Wyeth LLC. The authors are employees and shareholders of the Novartis group of companies, which are developing, manufacturing and marketing biopharmaceuticals, including biosimilar products.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

