Effect of Maternal Obesity on Maternal-Fetal Transfer of Preoperative Cefazolin at Cesarean Section
- PMID: 28638306
- PMCID: PMC5473397
- DOI: 10.5863/1551-6776-22.3.227
Effect of Maternal Obesity on Maternal-Fetal Transfer of Preoperative Cefazolin at Cesarean Section
Abstract
Objectives: American Congress of Obstetricians and Gynecologists recommends a single dose of antibiotic prophylaxis before all cesarean sections (C/S). This recommendation is based on pharmacokinetic studies that include only non-obese patients. We sought to evaluate 1) cefazolin plasma concentrations among obese and non-obese patients after administration of a 2-g cefazolin dose for prevention of surgical wound infections, and 2) whether cefazolin concentration in fetal circulation may be protective against pathogens that cause early onset neonatal sepsis.
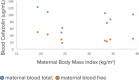
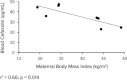
Methods: Maternal and fetal cefazolin plasma concentrations were compared between obese (body mass index [BMI] ≥ 30 kg/m2) and non-obese (BMI < 25 kg/m2) healthy, term pregnant women undergoing scheduled C/S. Liquid chromatographic-tandem mass spectrometric (LC-MS/MS) methods were used for quantification of total and free cefazolin concentrations in maternal blood (MB) and umbilical cord blood (UCB).
Results: Eight women were screened and consented. There was no difference between groups in MB total and free cefazolin concentrations. All MB samples had total and free cefazolin concentrations greater than the minimum inhibitory concentration 90 (MIC90) for Group B Streptococcus (GBS), Staphylococcus aureus, and Escherichia coli. All UCB samples had total and free cefazolin concentrations greater than MIC90 for GBS and S aureus, even when administered as briefly as 18 minutes before delivery. A lower concentration of total cefazolin was detected in UCB of neonates of obese women compared to non-obese women (p > 0.05).
Conclusions: Administration of 2 g of cefazolin to women undergoing scheduled C/S might be an adequate prophylactic dose for surgical wound infection in both non-obese and obese patients; and cefazolin concentration in fetal circulation may be protective against GBS and S aureus.
Keywords: antibiotic prophylaxis; cefazolin; obesity; pregnancy; surgical wound infection.
Conflict of interest statement
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Figures


Similar articles
-
Does current cefazolin dosing achieve adequate tissue and blood concentrations in obese women undergoing cesarean section?Eur J Obstet Gynecol Reprod Biol. 2017 Mar;210:334-341. doi: 10.1016/j.ejogrb.2017.01.022. Epub 2017 Jan 19. Eur J Obstet Gynecol Reprod Biol. 2017. PMID: 28122314
-
Prophylactic Cefazolin Dosing in Women With Body Mass Index >35 kg·m-2 Undergoing Cesarean Delivery: A Pharmacokinetic Study of Plasma and Interstitial Fluid.Anesth Analg. 2020 Jul;131(1):199-207. doi: 10.1213/ANE.0000000000004766. Anesth Analg. 2020. PMID: 32250982
-
Pharmacokinetics of cefazolin prophylaxis in obese gravidae at time of cesarean delivery.Am J Obstet Gynecol. 2015 Oct;213(4):541.e1-7. doi: 10.1016/j.ajog.2015.06.034. Epub 2015 Jun 20. Am J Obstet Gynecol. 2015. PMID: 26103528 Free PMC article. Clinical Trial.
-
Increased 3-gram cefazolin dosing for cesarean delivery prophylaxis in obese women.Am J Obstet Gynecol. 2015 Sep;213(3):415.e1-8. doi: 10.1016/j.ajog.2015.05.030. Epub 2015 May 21. Am J Obstet Gynecol. 2015. PMID: 26003059 Clinical Trial.
-
Lack of Pharmacokinetic Basis of Weight-Based Dosing and Intra-Operative Re-Dosing with Cefazolin Surgical Prophylaxis in Obese Patients: Implications for Antibiotic Stewardship.Surg Infect (Larchmt). 2019 Sep;20(6):439-443. doi: 10.1089/sur.2019.039. Epub 2019 May 21. Surg Infect (Larchmt). 2019. PMID: 31112072 Review.
Cited by
-
Maternal Group B Streptococcal Rectovaginal Colonization after Intrapartum Antibiotic Prophylaxis.Children (Basel). 2022 Nov 28;9(12):1848. doi: 10.3390/children9121848. Children (Basel). 2022. PMID: 36553292 Free PMC article.
-
Drugs for the Prevention and Treatment of Sepsis in the Newborn.Clin Perinatol. 2019 Jun;46(2):327-347. doi: 10.1016/j.clp.2019.02.012. Epub 2019 Mar 30. Clin Perinatol. 2019. PMID: 31010563 Free PMC article. Review.
-
Prophylactic perioperative cefuroxime levels in plasma and adipose tissue at the time of caesarean section (C-LACE): a protocol for a pilot experimental, prospective study with non-probability sampling to determine interpatient variability.Pilot Feasibility Stud. 2021 Feb 18;7(1):54. doi: 10.1186/s40814-021-00794-3. Pilot Feasibility Stud. 2021. PMID: 33602323 Free PMC article.
-
Prophylactic Cefazolin Dosing in Obesity-a Systematic Review.Obes Surg. 2022 Sep;32(9):3138-3149. doi: 10.1007/s11695-022-06196-5. Epub 2022 Jul 9. Obes Surg. 2022. PMID: 35809198 Free PMC article.
-
Maternal Obesity and Risk of Early-onset Neonatal Bacterial Sepsis: Nationwide Cohort and Sibling-controlled Studies.Clin Infect Dis. 2021 Nov 2;73(9):e2656-e2664. doi: 10.1093/cid/ciaa783. Clin Infect Dis. 2021. PMID: 32770206 Free PMC article.
References
-
- Barie PS. . Surgical site infections: epidemiology and prevention. Surg Infect (Larchmt). 2002; 3( suppl 1): S9– S21. - PubMed
-
- Pevzner L, Swank M, Krepel C, . et al. Effects of maternal obesity on tissue concentrations of prophylactic cefazolin during cesarean delivery. Obstet Gynecol. 2011; 117( 4): 877– 882. - PubMed
-
- Mivumbi VN, Little SE, Rulisa S, . et al. Prophylactic ampicillin versus cefazolin for the prevention of post-cesarean infectious morbidity in Rwanda. Int J Gynaecol Obstet. 2014; 124( 3): 244– 247. - PubMed
-
- Sarsam SE, Elliott JP, Lam GK. . Management of wound complications from cesarean delivery. Obstet Gynecol Surv. 2005; 60( 7): 462– 473. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
