Bile Acids Do Not Contribute to the Altered Calcium Homeostasis of Platelets from Rats with Biliary Cirrhosis
- PMID: 28638347
- PMCID: PMC5461275
- DOI: 10.3389/fphys.2017.00384
Bile Acids Do Not Contribute to the Altered Calcium Homeostasis of Platelets from Rats with Biliary Cirrhosis
Abstract
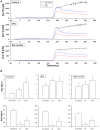
Previously, we have found that intracellular calcium homeostasis is altered in platelets from an experimental model of liver cirrhosis, the bile-duct ligated (BDL) rat; these alterations are compatible with the existence of a hypercoagulable state and related to an enhanced intracellular calcium release evoked by thrombin and an increased amount of calcium stored in the intracellular organelles. In the present study we have investigated the role of bile acids in those alterations of the BDL cirrhotic model. Cholic acid (CA) or deoxycholic acid (DCA) did not change P-selectin expression or platelet aggregation in any group but elevated baseline platelet calcium levels. Incubation with both bile acids reduced calcium release after stimulation with thrombin in the absence of extracellular calcium. Pretreatment with CA but not with DCA reduced significantly thrombin-induced calcium entry in all three experimental groups. The capacitative calcium entry was also significantly lower in platelets pretreated with both bile acids. The simultaneous addition of thapsigargin and ionomycin to estimate the total amount of calcium in platelet internal stores was decreased by pretreatment with both CA and DCA, although these changes were significantly different in the control rats only with CA and in the BDL platelets with DCA. These results indicate that CA and DCA reduce calcium movements in platelets of control and BDL animals, thus suggesting that bile acids do not participate in the alterations observed in the BDL cirrotic model.
Keywords: bile-duct ligation; calcium signaling; capacitative calcium entry; cholestasis; fura-2; liver cirrhosis; thapsigargin; thrombin.
Figures


References
-
- Annie-Jeyachristy S., Geetha A., Surendran R. (2008). Changes in the level of cytosolic calcium, nitric oxide and nitric oxide synthase activity during platelet aggregation: an in vitro study in platelets from normal subjects and those with cirrhosis. J. Biosci. 33, 45–53. 10.1007/s12038-008-0020-0 - DOI - PubMed
-
- Baele G., Beke R., Barbier F. (1980). In vitro inhibition of platelet aggregation by bile salts. Thromb. Haemost. 44, 62–64. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

