Candida albicans Yeast, Pseudohyphal, and Hyphal Morphogenesis Differentially Affects Immune Recognition
- PMID: 28638380
- PMCID: PMC5461353
- DOI: 10.3389/fimmu.2017.00629
Candida albicans Yeast, Pseudohyphal, and Hyphal Morphogenesis Differentially Affects Immune Recognition
Abstract
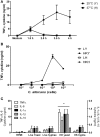
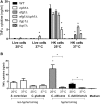
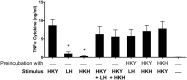
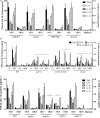
Candida albicans is a human opportunist pathogen that can grow as yeast, pseudohyphae, or true hyphae in vitro and in vivo, depending on environmental conditions. Reversible cellular morphogenesis is an important virulence factor that facilitates invasion of host tissues, escape from phagocytes, and dissemination in the blood stream. The innate immune system is the first line of defense against C. albicans infections and is influenced by recognition of wall components that vary in composition in different morphological forms. However, the relationship between cellular morphogenesis and immune recognition of this fungus is not fully understood. We therefore studied various vegetative cell types of C. albicans, singly and in combination, to assess the consequences of cellular morphogenesis on selected immune cytokine outputs from human monocytes. Hyphae stimulated proportionally lower levels of certain cytokines from monocytes per unit of cell surface area than yeast cells, but did not suppress cytokine response when copresented with yeast cells. Pseudohyphal cells induced intermediate cytokine responses. Yeast monomorphic mutants had elevated cytokine responses under conditions that otherwise supported filamentous growth and mutants of yeast and hyphal cells that were defective in cell wall mannosylation or lacking certain hypha-specific cell wall proteins could variably unmask or deplete the surface of immunostimulatory ligands. These observations underline the critical importance of C. albicans morphology and morphology-associated changes in the cell wall composition that affect both immune recognition and pathogenesis.
Keywords: Candida albicans; cell wall; cytokine; immune recognition; morphogenesis.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

