Convenient and General Zinc-Catalyzed Borylation of Aryl Diazonium Salts and Aryltriazenes under Mild Conditions
- PMID: 28638765
- PMCID: PMC5474665
- DOI: 10.1002/open.201700036
Convenient and General Zinc-Catalyzed Borylation of Aryl Diazonium Salts and Aryltriazenes under Mild Conditions
Abstract
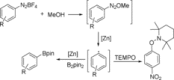
A convenient and general zinc-catalyzed borylation of aryl diazonium salts and aryltriazenes has been developed. With bis- (pinacolato)diboron as the borylation reagent, aryldiazonium tetrafluoroborate salts and aryltriazenes were transformed into the corresponding arylboronates in moderate to excellent yields under mild conditions. As a convenient and practical methodology, no additional ligands, base, or any other additives are required here.
Keywords: arylboronates; aryldiazonium salts; aryltriazenes; borylation; zinc catalysis.
Figures
References
-
- None
-
- Rudolph A., Lautens M., Angew. Chem. Int. Ed. 2009, 48, 2656–2670; - PubMed
- Angew. Chem. 2009, 121, 2694–2708;
-
- Imao D., Glasspoole B. W., Laberge V. S., Crudden C. M., J. Am. Chem. Soc. 2009, 131, 5024–5025; - PubMed
-
- Frisch A. C., Beller M., Angew. Chem. Int. Ed. 2005, 44, 674–688; - PubMed
- Angew. Chem. 2005, 117, 680–695;
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous