Extracellular Vesicles in Renal Pathophysiology
- PMID: 28638822
- PMCID: PMC5461431
- DOI: 10.3389/fmolb.2017.00037
Extracellular Vesicles in Renal Pathophysiology
Abstract
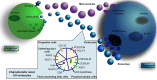
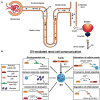
Extracellular vesicles are a heterogeneous population of microparticles released by virtually all living cells which have been recently widely investigated in different biological fields. They are typically composed of two primary types (exosomes and microvesicles) and are recently commanding increasing attention as mediators of cellular signaling. Indeed, these vesicles can affect recipient cells by carrying and delivering complex cargos of biomolecules (including proteins, lipids and nucleic acids), protected from enzymatic degradation in the environment. Their importance has been demonstrated in the pathophysiology of several organs, in particular in kidney, where different cell types secrete extracellular vesicles that mediate their communication with downstream urinary tract cells. Over the past few years, evidence has been shown that vesicles participate in kidney development and normal physiology. Moreover, EVs are widely demonstrated to be implicated in cellular signaling during renal regenerative and pathological processes. Although many EV mechanisms are still poorly understood, in particular in kidney, the discovery of their role could help to shed light on renal biological processes which are so far elusive. Lastly, extracellular vesicles secreted by renal cells gather in urine, thus becoming a great resource for disease or recovery markers and a promising non-invasive diagnostic instrument for renal disease. In the present review, we discuss the most recent findings on the role of extracellular vesicles in renal physiopathology and their potential implication in diagnosis and therapy.
Keywords: biomarkers; biomolecules; extracellular vesicles; intercellular communication; kidney; pathology; physiology.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

