Parietal white matter lesions in Alzheimer's disease are associated with cortical neurodegenerative pathology, but not with small vessel disease
- PMID: 28638989
- PMCID: PMC5563333
- DOI: 10.1007/s00401-017-1738-2
Parietal white matter lesions in Alzheimer's disease are associated with cortical neurodegenerative pathology, but not with small vessel disease
Abstract
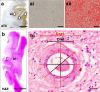
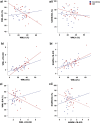
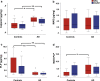
Cerebral white matter lesions (WML) encompass axonal loss and demyelination, and the pathogenesis is assumed to be small vessel disease (SVD)-related ischemia. However, WML may also result from the activation of Wallerian degeneration as a consequence of cortical Alzheimer's disease (AD) pathology, i.e. hyperphosphorylated tau (HPτ) and amyloid-beta (Aβ) deposition. WML seen in AD have a posterior predominance compared to non-demented individuals but it is unclear whether the pathological and molecular signatures of WML differ between these two groups. We investigated differences in the composition and aetiology of parietal WML from AD and non-demented controls. Parietal WML tissue from 55 human post-mortem brains (AD, n = 27; non-demented controls, n = 28) were quantitatively assessed for axonal loss and demyelination, as well as for cortical HPτ and Aβ burden and SVD. Biochemical assessment included Wallerian degeneration protease calpain and the myelin-associated glycoprotein (MAG) to proteolipid protein (PLP) ratio (MAG:PLP) as a measure of hypoperfusion. WML severity was associated with both axonal loss and demyelination in AD, but only with demyelination in controls. Calpain was significantly increased in WML tissue in AD, whereas MAG:PLP was significantly reduced in controls. Calpain levels were associated with increasing amounts of cortical AD-pathology but not SVD. We conclude that parietal WML seen in AD differ in their pathological composition and aetiology compared to WML seen in aged controls: WML seen in AD may be associated with Wallerian degeneration that is triggered by cortical AD-pathology, whereas WML in aged controls are due to ischaemia. Hence, parietal WML as seen on MRI should not invariably be interpreted as a surrogate biomarker for SVD as they may be indicative of cortical AD-pathology, and therefore, AD should also be considered as the main underlying cause for cognitive impairment in cases with parietal WML.
Keywords: Alzheimer’s disease; Hyperphosphorylated tau; Small vessel disease; Wallerian degeneration; White matter hyperintensity; White matter lesion.
Conflict of interest statement
Funding
The research was supported by the Alzheimer’s Society (Grant Number: AS-PG-2013-011). Tissue for this study was provided by the Newcastle Brain Tissue Resource, which is funded in part by a grant from the UK Medical Research Council (G0400074) and by Brains for Dementia research, a joint venture between Alzheimer’s Society and Alzheimer’s Research UK.
Conflict of interest
The authors declare that they have no conflict of interest.
Figures


References
-
- Barber R, Scheltens P, Gholkar A, Ballard C, McKeith I, Ince P, et al. White matter lesions on magnetic resonance imaging in dementia with Lewy bodies, Alzheimer’s disease, vascular dementia, and normal aging. J Neurol Neurosurg Psychiatry. 1999;67:66–72. doi: 10.1136/jnnp.67.1.66. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

