Rigidity of the extracellular part of HER2: Evidence from engineering subdomain interfaces and shared-helix DARPin-DARPin fusions
- PMID: 28639341
- PMCID: PMC5563139
- DOI: 10.1002/pro.3216
Rigidity of the extracellular part of HER2: Evidence from engineering subdomain interfaces and shared-helix DARPin-DARPin fusions
Abstract
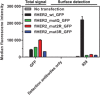
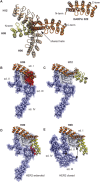
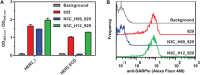
The second member of the human ErbB family of receptor tyrosine kinases, HER2/hErbB2, is regarded as an exceptional case: The four extracellular subdomains could so far only be found in one fixed overall conformation, designated "open" and resembling the ligand-bound form of the other ErbB receptors. It thus appears to be different from the extracellular domains of the other family members that show inter-subdomain flexibility and exist in a "tethered" form in the absence of ligand. For HER2, there was so far no direct evidence for such a tethered conformation on the cell surface. Nonetheless, alternative conformations of HER2 in vivo could so far not be excluded. We now demonstrate the rigidity of HER2 on the surface of tumor cells by employing two orthogonal approaches of protein engineering: To directly test the potential of the extracellular domain of HER2 to adopt a pseudo-tethered conformation on the cell surface, we first designed HER2 variants with a destabilized interface between extracellular subdomains I and III that would favor deviation from the "open" conformation. Secondly, we used differently shaped versions of a Designed Ankyrin Repeat Protein (DARPin) fusion, recognizing subdomain I of HER2, devised to work as probes for a putative pseudo-tethered extracellular domain of HER2. Combining our approaches, we exclude, on live cells and in vitro, that significant proportions of HER2 deviate from the "open" conformation.
Keywords: DARPins; ErbB receptors; HER2; conformational probe; protein engineering.
© 2017 The Protein Society.
Figures

References
-
- Baselga J, Swain SM (2009) Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer 9:463–475. - PubMed
-
- Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, Jr , Leahy DJ (2003) Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 421:756–760. - PubMed
-
- Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX (2004) Insights into ErbB signaling from the structure of the ErbB2‐pertuzumab complex. Cancer Cell 5:317–328. - PubMed
-
- Garrett TPJ, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO, Kofler M, Jorissen RN, Nice EC, Burgess AW, Ward CW (2003) The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol Cell 11:495–505. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

