Topical Delivery of Immunosuppression to Prolong Xenogeneic and Allogeneic Split-Thickness Skin Graft Survival
- PMID: 28639977
- PMCID: PMC5720930
- DOI: 10.1097/BCR.0000000000000597
Topical Delivery of Immunosuppression to Prolong Xenogeneic and Allogeneic Split-Thickness Skin Graft Survival
Abstract
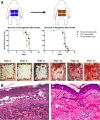
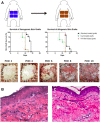
Cadaveric skin allograft is the current standard of treatment for temporary coverage of large burn wounds. Porcine xenografts are viable alternatives but undergo α-1,3-galactose (Gal)-mediated hyperacute rejection and are lost by post-operative day (POD) 3 because of naturally occurring antibodies to Gal in primate recipients. Using baboons, we previously demonstrated that xenografts from GalT-KO swine (lacking Gal) provided wound coverage comparable with allografts with systemic immunosuppression. In this study, we investigate topical immunosuppression as an alternative to prolong xenograft survival. Full-thickness wounds in baboons were created and covered with xenogeneic and allogeneic split-thickness skin grafts (STSGs). Animals were treated with slow-release (TyroSphere-encapsulated) topical formulations (cyclosporine-A [CSA] or Tacrolimus) applied 1) directly to the STSGs only, or 2) additionally to the wound bed before STSG and 1). Topical CSA did not improve either xenograft or allograft survival (median: treated grafts = 12.5 days, control = 14 days; P = 0.27) with similar results when topical Tacrolimus was used. Pretreatment of wound beds resulted in a significant reduction of xenograft survival compared with controls (10 vs 14 days; P = 0.0002), with comparable results observed in allografts. This observation was associated with marked reduction of inflammation on histology with Tacrolimus and not CSA. Prolongation of allograft and xenograft survival after application to full-thickness wound beds was not achieved with the current formulation of topical immunosuppressants. Modulation of inflammation within the wound bed was effective with Tacrolimus pretreatment before STSG application and may serve as a treatment strategy in related fields.
Figures

References
-
- Grunwald TB, Garner WL. Acute burns. Plast Reconstr Surg 2008;121:311e–9e. - PubMed
-
- Sheridan RL. Burn care: results of technical and organizational progress. JAMA 2003;290:719–22. - PubMed
-
- Garner WL, Magee W. Acute burn injury. Clin Plast Surg 2005;32:187–93. - PubMed
-
- Sheridan RL. Comprehensive treatment of burns. Curr Probl Surg 2001;38:657–756. - PubMed
-
- Greenhalgh DG, Saffle JR, Holmes JH 4th, et al. American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res 2007;28(6):776–790. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

