Reduction in Diarrhea- and Rotavirus-related Healthcare Visits Among Children <5 Years of Age After National Rotavirus Vaccine Introduction in Zimbabwe
- PMID: 28640001
- PMCID: PMC5600692
- DOI: 10.1097/INF.0000000000001648
Reduction in Diarrhea- and Rotavirus-related Healthcare Visits Among Children <5 Years of Age After National Rotavirus Vaccine Introduction in Zimbabwe
Abstract
Background: In Zimbabwe, rotavirus accounted for 41%-56% of acute diarrhea hospitalizations before rotavirus vaccine introduction in 2014. We evaluated rotavirus vaccination impact on acute diarrhea- and rotavirus-related healthcare visits in children.
Methods: We examined monthly and annual acute diarrhea and rotavirus test-positive hospitalizations and Accident and Emergency Department visits among children <60 months of age at 3 active surveillance hospitals during 2012-2016; we compared prevaccine introduction (2012-2013) with postvaccine introduction (2015 and 2016) data for 2 of the hospitals. We examined monthly acute diarrhea hospitalizations by year and age group for 2013-2016 from surveillance hospital registers and monthly acute diarrhea outpatient visits reported to the Ministry of Health and Child Care during 2012-2016.
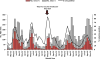
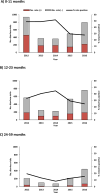
Results: Active surveillance data showed winter seasonal peaks in diarrhea- and rotavirus-related visits among children <60 months of age during 2012-2014 that were substantially blunted in 2015 and 2016 after vaccine introduction; the percentage of rotavirus test-positive visits followed a similar seasonal pattern and decrease. Hospital register data showed similar pre-introduction seasonal variation and post-introduction declines in diarrhea hospitalizations among children 0-11 and 12-23 months of age. Monthly variation in outpatient diarrhea-related visits mirrored active surveillance data patterns. At 2 surveillance hospitals, the percentage of rotavirus-positive visits declined by 40% and 43% among children 0-11 months of age and by 21% and 33% among children 12-23 months of age in 2015 and 2016, respectively.
Conclusion: Initial reductions in diarrheal illness among children <60 months of age, particularly among those 0-11 months of age, after vaccine introduction are encouraging. These early results provide evidence to support continued rotavirus vaccination and rotavirus surveillance in Zimbabwe.
Conflict of interest statement
Figures
References
-
- UNICEF. One is too many: Ending child deaths from pneumonia and diarrhoea. 2016
-
- World Health Organization. Rotavirus Vaccines WHO Position Paper. Weekly Epidemiological Record. 2013;88(5):49–64. - PubMed
-
- World Health Organization. Rotavirus vaccines: an update. Weekly Epidemiological Record. 2009;84(51):533–40. - PubMed
-
- Mukaratirwa A, Berejena C, Nziramasanga P, et al. Epidemiologic and genotypic characteristics of rotavirus strains detected in children less than 5 years of age with gastroenteritis treated at 3 pediatric hospitals in Zimbabwe during 2008–2011. Pediatr Infect Dis J. 2014;33(Suppl 1):S45–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

