An R-CaMP1.07 reporter mouse for cell-type-specific expression of a sensitive red fluorescent calcium indicator
- PMID: 28640817
- PMCID: PMC5480891
- DOI: 10.1371/journal.pone.0179460
An R-CaMP1.07 reporter mouse for cell-type-specific expression of a sensitive red fluorescent calcium indicator
Abstract
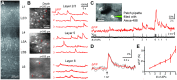
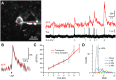
Genetically encoded calcium indicators (GECIs) enable imaging of in vivo brain cell activity with high sensitivity and specificity. In contrast to viral infection or in utero electroporation, indicator expression in transgenic reporter lines is induced noninvasively, reliably, and homogenously. Recently, Cre/tTA-dependent reporter mice were introduced, which provide high-level expression of green fluorescent GECIs in a cell-type-specific and inducible manner when crossed with Cre and tTA driver mice. Here, we generated and characterized the first red-shifted GECI reporter line of this type using R-CaMP1.07, a red fluorescent indicator that is efficiently two-photon excited above 1000 nm. By crossing the new R-CaMP1.07 reporter line to Cre lines driving layer-specific expression in neocortex we demonstrate its high fidelity for reporting action potential firing in vivo, long-term stability over months, and versatile use for functional imaging of excitatory neurons across all cortical layers, especially in the previously difficult to access layers 4 and 6.
Conflict of interest statement
Figures



References
-
- Grienberger C, Konnerth A. Imaging calcium in neurons. Neuron. 2012;73(5):862–85. doi: 10.1016/j.neuron.2012.02.011 . - DOI - PubMed
-
- Lin MZ, Schnitzer MJ. Genetically encoded indicators of neuronal activity. Nature Neuroscience. 2016;19(9):1142–53. doi: 10.1038/nn.4359 . - DOI - PMC - PubMed
-
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nature Methods. 2009;6(12):875–81. doi: 10.1038/nmeth.1398 - DOI - PMC - PubMed
-
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499(7458):295–300. doi: 10.1038/nature12354 ; - DOI - PMC - PubMed
-
- Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nature Biotechnology. 2001;19(2):137–41. doi: 10.1038/84397 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

