Toxic effects of atrazine on porcine oocytes and possible mechanisms of action
- PMID: 28640859
- PMCID: PMC5480989
- DOI: 10.1371/journal.pone.0179861
Toxic effects of atrazine on porcine oocytes and possible mechanisms of action
Abstract
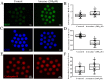
Because atrazine is a widely used herbicide, its adverse effects on the reproductive system have been extensively researched. In this study, we investigated the effects of atrazine exposure on porcine oocyte maturation and the possible mechanisms. Our results showed that the rates of oocyte maturation significantly decreased after treatment with 200 μM atrazine in vitro. Atrazine treatment resulted in abnormal spindle morphology but did not affect actin distribution. Atrazine exposure not only triggered a DNA damage response but also decreased MPF levels in porcine oocytes. Our results also revealed that atrazine worsened porcine oocyte quality by causing excessive accumulation of superoxide radicals, increasing cathepsin B activity, and decreasing the GSH level and mitochondrial membrane potential. Furthermore, atrazine decreased developmental competence of porcine oocytes up to the blastocyst stage and changed some properties: cell numbers, apoptosis, and related gene expression levels. Collectively, our results indicate that porcine oocyte maturation is defective after atrazine treatment at least through disruption of spindle morphology, MPF activity, and mitochondrial function and via induction of DNA damage, which probably reduces developmental competence.
Conflict of interest statement
Figures



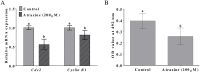


References
-
- Belzunces B, Hoyau S, Benoit M, Tarrat N, Bessac F. Theoretical study of the atrazine pesticide interaction with pyrophyllite and Ca2+ -montmorillonite clay surfaces. Journal of computational chemistry. 2017;38(3):133–43. doi: 10.1002/jcc.24530 . - DOI - PubMed
-
- Chromcova L, Blahova J, Plhalova L, Zivna D, Stepanova S, Praskova E, et al. The effects of atrazine exposure on early life stages of common carp (Cyprinus carpio). Neuro endocrinology letters. 2013;34 Suppl 2:95–101. . - PubMed
-
- Sass JB, Colangelo A. European Union bans atrazine, while the United States negotiates continued use. International journal of occupational and environmental health. 2006;12(3):260–7. doi: 10.1179/oeh.2006.12.3.260 . - DOI - PubMed
-
- Traba HM, Dominguez-Morueco N, Barreno E, Catala M. Lichen microalgae are sensitive to environmental concentrations of atrazine. Journal of environmental science and health Part B, Pesticides, food contaminants, and agricultural wastes. 2017:1–6. doi: 10.1080/03601234.2016.1270679 . - DOI - PubMed
-
- Hayes TB, Khoury V, Narayan A, Nazir M, Park A, Brown T, et al. Atrazine induces complete feminization and chemical castration in male African clawed frogs (Xenopus laevis). Proceedings of the National Academy of Sciences of the United States of America. 2010;107(10):4612–7. doi: 10.1073/pnas.0909519107 ; - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

