Targeting breast cancer cells by MRS1477, a positive allosteric modulator of TRPV1 channels
- PMID: 28640864
- PMCID: PMC5481018
- DOI: 10.1371/journal.pone.0179950
Targeting breast cancer cells by MRS1477, a positive allosteric modulator of TRPV1 channels
Abstract
There is convincing epidemiological and experimental evidence that capsaicin, a potent natural transient receptor potential cation channel vanilloid member 1 (TRPV1) agonist, has anticancer activity. However, capsaicin cannot be given systemically in large doses, because of its induction of acute pain and neurological inflammation. MRS1477, a dihydropyridine derivative acts as a positive allosteric modulator of TRPV1, if added together with capsaicin, but is ineffective, if given alone. Addition of MRS1477 evoked Ca2+ signals in MCF7 breast cancer cells, but not in primary breast epithelial cells. This indicates that MCF7 cells not only express functional TRPV1 channels, but also produce endogenous TRPV1 agonists. We investigated the effects of MRS1477 and capsaicin on cell viability, caspase-3 and -9 activities and reactive oxygen species production in MCF7 cells. The fraction of apoptotic cells was increased after 3 days incubation with capsaicin (10 μM) paralleled by increased reactive oxygen species production and caspase activity. These effects were even more pronounced, when cells were incubated with MRS1477 (2 μM) either alone or together with CAPS (10 μM). Capsazepine, a TRPV1 blocker, inhibited both the effect of capsaicin and MRS1477. Whole-cell patch clamp recordings revealed that capsaicin-evoked TRPV1-mediated current density levels were increased after 3 days incubation with MRS1477 (2 μM). However, the tumor growth in MCF7 tumor-bearing immunodeficient mice was not significantly decreased after treatment with MRS1477 (10 mg/ kg body weight, i.p., injection twice a week). In conclusion, in view of a putative in vivo treatment with MRS1477 or similar compounds further optimization is required.
Conflict of interest statement
Figures

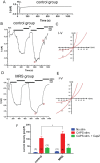
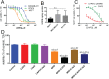
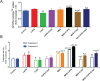
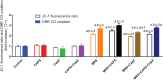

References
-
- Schafer M, Werner S (2008) Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol 9: 628–638. doi: 10.1038/nrm2455 - DOI - PubMed
-
- Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140: 883–899. doi: 10.1016/j.cell.2010.01.025 - DOI - PMC - PubMed
-
- Barar J (2012) Targeting tumor microenvironment: the key role of immune system. Bioimpacts 2: 1–3. doi: 10.5681/bi.2012.001 - DOI - PMC - PubMed
-
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21: 531–543. - PubMed
-
- Olah Z, Karai L, Iadarola MJ (2001) Anandamide activates vanilloid receptor 1 (VR1) at acidic pH in dorsal root ganglia neurons and cells ectopically expressing VR1. J Biol Chem 276: 31163–31170. doi: 10.1074/jbc.M101607200 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

